Difference between revisions of "Cerulean blue"
| (6 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:Cerulean blue C100x.jpg|thumb|Cerulean blue]] | [[File:Cerulean blue C100x.jpg|thumb|Cerulean blue]] | ||
== Description == | == Description == | ||
| − | + | [[File:43_Cerulean_blue_500X.jpg|thumb|Cerulean blue at 500x]] | |
| + | [[File:43_Cerulean_blue_500X_pol.jpg|thumb|Cerulean blue at 500x polarized light]] | ||
A synthetic sky blue pigment originally composed of cobalt magnesium stannate. Cerulean blue was perfected by a process developed by Andreas Höpfner in Germany in 1805 that roasted cobalt and tin oxides. However, the color was not sold as an artist pigment until 1860 by Rowney and Company under the name of coeruleum (Gettens and Stout 1966). Cerulean blue is an inert, lightfast pigment that acts as a drier for oil paints. Some commercial color formulations sold as the cerulean blue hue include: | A synthetic sky blue pigment originally composed of cobalt magnesium stannate. Cerulean blue was perfected by a process developed by Andreas Höpfner in Germany in 1805 that roasted cobalt and tin oxides. However, the color was not sold as an artist pigment until 1860 by Rowney and Company under the name of coeruleum (Gettens and Stout 1966). Cerulean blue is an inert, lightfast pigment that acts as a drier for oil paints. Some commercial color formulations sold as the cerulean blue hue include: | ||
| Line 13: | Line 14: | ||
- zinc oxide and phthalocyanine | - zinc oxide and phthalocyanine | ||
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 20: | Line 19: | ||
cobalt magnesium stannate; Pigment Blue 35; CI 77346; CI 77368; azul cerúleo (Esp.); Coelinblau (Deut.); bleu ciel (Fr.); bleu ceruleum (Fr.); blu ceruleo (It.); ceruleum blauw (Ned.); azul cerúleo (Port.); cobaltous stannate; cobalt tin oxide; coelin; ceruleum blue; caeruleum; coeruleum; magnesium cerulean blue | cobalt magnesium stannate; Pigment Blue 35; CI 77346; CI 77368; azul cerúleo (Esp.); Coelinblau (Deut.); bleu ciel (Fr.); bleu ceruleum (Fr.); blu ceruleo (It.); ceruleum blauw (Ned.); azul cerúleo (Port.); cobaltous stannate; cobalt tin oxide; coelin; ceruleum blue; caeruleum; coeruleum; magnesium cerulean blue | ||
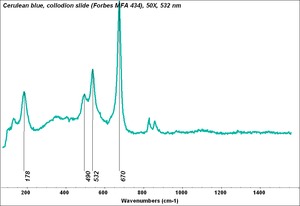
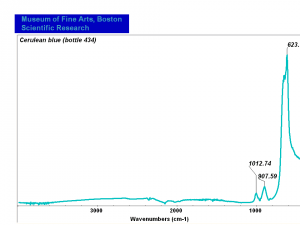
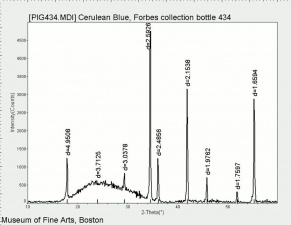
| − | [[[SliderGallery rightalign| | + | [[[SliderGallery rightalign|Cerulean blue, collodion slide (Forbes MFA 434), 50X, 532 nm.TIF~Raman (MFA)|Cerulean blue (bottle 434).PNG~FTIR (MFA)|PIG434.jpg~XRD (MFA)|f434sem.jpg~SEM|f434edsbw.jpg~EDS]]] |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | ==Physical and Chemical Properties== |
| − | + | * Very fine, rounded, isotropic, greenish-blue particles. | |
| + | * High refractive index. | ||
| + | * No birefringence. | ||
| + | * No pleochroism. | ||
| + | * Appears deep red through Chelsea filter. | ||
| + | * Inert to acids and bases. | ||
| + | * Composition = CoO-nSnO2 | ||
| + | * Refractive Index = 1.78 - 1.84 | ||
| − | == | + | == Risks == |
| − | + | * Skin contact may cause allergies. | |
| + | * Chronic inhalation may cause asthma and possible fibrosis. | ||
| + | * Ingestion may cause acute vomiting and diarrhea. | ||
== Comparisons == | == Comparisons == | ||
| Line 51: | Line 42: | ||
[[media:download_file_486.pdf|Characteristics of Common Blue Pigments]] | [[media:download_file_486.pdf|Characteristics of Common Blue Pigments]] | ||
| + | ==Resources and Citations== | ||
| − | + | * Michel Bouchard, Alessa Gambardella, 'Raman microscopy study of synthetic cobalt blue spinels used in the field of art', ''J. Raman Spectroscopy'' (2010) at http://www.interscience.wiley.com/journal/jrs. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * Michel Bouchard, Alessa Gambardella, 'Raman microscopy study of synthetic cobalt blue spinels used in the field of art', ''J. Raman Spectroscopy'' (2010) at | ||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| Line 84: | Line 66: | ||
* David Bomford, Jo Kirby, John Leighton, Ashok Roy, ''Art in the Making:Impressionism'', National Gallery, London, 1990. Comment: introduced as water color pigment by Rowney in 1860 | * David Bomford, Jo Kirby, John Leighton, Ashok Roy, ''Art in the Making:Impressionism'', National Gallery, London, 1990. Comment: introduced as water color pigment by Rowney in 1860 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Cerulean (Accessed Sept 2 2005) |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * | + | * Pigments Through the Ages: http://webexhibits.org/pigments/indiv/overview/ceruleanblue.html gives "reintroduction in 1860 by George Rowney in England" |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 09:49, 24 May 2022
Description
A synthetic sky blue pigment originally composed of cobalt magnesium stannate. Cerulean blue was perfected by a process developed by Andreas Höpfner in Germany in 1805 that roasted cobalt and tin oxides. However, the color was not sold as an artist pigment until 1860 by Rowney and Company under the name of coeruleum (Gettens and Stout 1966). Cerulean blue is an inert, lightfast pigment that acts as a drier for oil paints. Some commercial color formulations sold as the cerulean blue hue include:
- oxides of cobalt and aluminum
- cobalt chromate
- oxides of cobalt, aluminum, and chromium
- titanium dioxide and phthalocyanine
- zinc oxide and phthalocyanine
Synonyms and Related Terms
cobalt magnesium stannate; Pigment Blue 35; CI 77346; CI 77368; azul cerúleo (Esp.); Coelinblau (Deut.); bleu ciel (Fr.); bleu ceruleum (Fr.); blu ceruleo (It.); ceruleum blauw (Ned.); azul cerúleo (Port.); cobaltous stannate; cobalt tin oxide; coelin; ceruleum blue; caeruleum; coeruleum; magnesium cerulean blue
Physical and Chemical Properties
- Very fine, rounded, isotropic, greenish-blue particles.
- High refractive index.
- No birefringence.
- No pleochroism.
- Appears deep red through Chelsea filter.
- Inert to acids and bases.
- Composition = CoO-nSnO2
- Refractive Index = 1.78 - 1.84
Risks
- Skin contact may cause allergies.
- Chronic inhalation may cause asthma and possible fibrosis.
- Ingestion may cause acute vomiting and diarrhea.
Comparisons
Characteristics of Common Blue Pigments
Resources and Citations
- Michel Bouchard, Alessa Gambardella, 'Raman microscopy study of synthetic cobalt blue spinels used in the field of art', J. Raman Spectroscopy (2010) at http://www.interscience.wiley.com/journal/jrs.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966. Comment: first sold in 1860
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971. Comment: p. 810
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996. Comment: "Pigment"
- M. Doerner, The Materials of the Artist, Harcourt, Brace & Co., 1934.
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983.
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing. Comment: introduced as artist pigment in 1870
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979.
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980.
- David Bomford, Jo Kirby, John Leighton, Ashok Roy, Art in the Making:Impressionism, National Gallery, London, 1990. Comment: introduced as water color pigment by Rowney in 1860
- Wikipedia: http://en.wikipedia.org/wiki/Cerulean (Accessed Sept 2 2005)
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Pigments Through the Ages: http://webexhibits.org/pigments/indiv/overview/ceruleanblue.html gives "reintroduction in 1860 by George Rowney in England"