Difference between revisions of "Chlorotoluene"
Jump to navigation
Jump to search
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A colorless, low-viscosity [[solvent]] used for [[ | + | A colorless, low-viscosity [[solvent]] used for [[Rubber (natural, vulcanized)|rubber]] and [[synthetic resin|synthetic resins]]. It is also used as an intermediate in the manufacture of [[dye|dyes]]. |
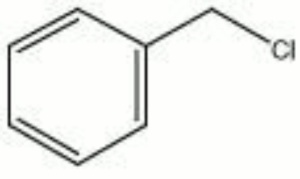
| − | + | [[[SliderGallery rightalign|chlorotoluene.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
monochlorotoluene; alpha-chlorotoluene; benzyl chloride; chlorophenylmethane; chloromethylbenzene | monochlorotoluene; alpha-chlorotoluene; benzyl chloride; chlorophenylmethane; chloromethylbenzene | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Highly toxic by inhalation. | ||
| + | * Strong irritation. | ||
| + | * May be absorbed through the skin. | ||
| + | * Combustible. Flash point = 67C | ||
| + | * Burning produces toxic fumes. | ||
| + | * Sigma-Aldrich: [https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=138509&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F138509%3Flang%3Den SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Miscible in ethanol, acetone, ether, benzene, carbon tetrachloride and heptane. Slightly soluble in water. | Miscible in ethanol, acetone, ether, benzene, carbon tetrachloride and heptane. Slightly soluble in water. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -43 | + | | -43 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.0776 | + | | 1.0776 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 179 | + | | 179 C |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 819 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 819 | ||
Latest revision as of 13:43, 29 May 2022
Description
A colorless, low-viscosity Solvent used for rubber and synthetic resins. It is also used as an intermediate in the manufacture of dyes.
Synonyms and Related Terms
monochlorotoluene; alpha-chlorotoluene; benzyl chloride; chlorophenylmethane; chloromethylbenzene
Risks
- Highly toxic by inhalation.
- Strong irritation.
- May be absorbed through the skin.
- Combustible. Flash point = 67C
- Burning produces toxic fumes.
- Sigma-Aldrich: SDS
Physical and Chemical Properties
Miscible in ethanol, acetone, ether, benzene, carbon tetrachloride and heptane. Slightly soluble in water.
| Composition | CH3C6H4Cl |
|---|---|
| CAS | 100-44-7 |
| Melting Point | -43 C |
| Density | 1.0776 g/ml |
| Molecular Weight | mol. wt. = 126.6 |
| Boiling Point | 179 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 819
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979