Difference between revisions of "Clay"
(username removed) |
|||
| (6 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:11.3009-C34312CR-d1.jpg|thumb|]] | + | [[File:11.3009-C34312CR-d1.jpg|thumb|Egyptian jar<br>MFA# 11.3009]] |
== Description == | == Description == | ||
| − | A naturally occurring earthy mineral that is plastic when wet, reversibly hard when dry, and permanently hard when heated. Clays are formed by the weathering of feldspathic rock. Primary clays are found at the site of the parent stone while secondary clays are found downstream. Clays are composed of hydrated aluminum silicates, such as [ | + | A naturally occurring earthy mineral that is plastic when wet, reversibly hard when dry, and permanently hard when heated. Clays are formed by the weathering of feldspathic rock. Primary clays are found at the site of the parent stone while secondary clays are found downstream. Clays are composed of hydrated aluminum silicates, such as [[kaolinite]], [[illite]], [[palygorskite]], [[attapulgite]], [[bentonite]], and [[montmorillonite]]. Small amounts of other minerals can change the color (white, yellow, brown or red) and texture of the clays. When pure, clay is a fine, white, amorphous powder which becomes plastic when water is added. At high temperatures, clays became hard due to the loss of water and are used to make pottery, porcelain, and brick. Clay is also used as a filler and whiting in paper, paint, and grounds. |
| + | |||
| + | [[File:1989.159-SC39738.jpg|thumb|Persian seal<br>MFA# 1989.159]] | ||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
clays; China clay; kaolin; porcelain clay; tonerde; fuller's earth; white bole; pipe clay; Bouvigal white; Rouen white; Spanish white; Troy white; feldspar; arcilla (Esp.); argile (Fr.); argila (Port.); Ton (Deut.); klei (Ned.) | clays; China clay; kaolin; porcelain clay; tonerde; fuller's earth; white bole; pipe clay; Bouvigal white; Rouen white; Spanish white; Troy white; feldspar; arcilla (Esp.); argile (Fr.); argila (Port.); Ton (Deut.); klei (Ned.) | ||
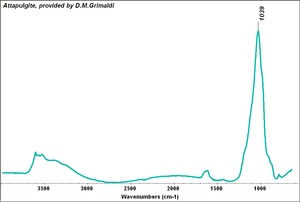
| − | == | + | [[[SliderGallery rightalign|Attapulgite, Grimaldi.TIF~FTIR (MFA)]]] |
| + | |||
| + | == Risks == | ||
| + | |||
| + | Dusts may irritate nose and throat. | ||
| + | == Physical and Chemical Properties == | ||
Particle size 1 to 150 micrometers. Insoluble in water and organic solvents | Particle size 1 to 150 micrometers. Insoluble in water and organic solvents | ||
| Line 19: | Line 25: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | about 2.5 | + | | about 2.5 g/ml |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 174 |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Clay." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Clay." Accessed 11 Sep, 2003 . |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
Latest revision as of 10:31, 30 May 2022
Description
A naturally occurring earthy mineral that is plastic when wet, reversibly hard when dry, and permanently hard when heated. Clays are formed by the weathering of feldspathic rock. Primary clays are found at the site of the parent stone while secondary clays are found downstream. Clays are composed of hydrated aluminum silicates, such as Kaolinite, Illite, Palygorskite, Attapulgite, Bentonite, and Montmorillonite. Small amounts of other minerals can change the color (white, yellow, brown or red) and texture of the clays. When pure, clay is a fine, white, amorphous powder which becomes plastic when water is added. At high temperatures, clays became hard due to the loss of water and are used to make pottery, porcelain, and brick. Clay is also used as a filler and whiting in paper, paint, and grounds.
Synonyms and Related Terms
clays; China clay; kaolin; porcelain clay; tonerde; fuller's earth; white bole; pipe clay; Bouvigal white; Rouen white; Spanish white; Troy white; feldspar; arcilla (Esp.); argile (Fr.); argila (Port.); Ton (Deut.); klei (Ned.)
Risks
Dusts may irritate nose and throat.
Physical and Chemical Properties
Particle size 1 to 150 micrometers. Insoluble in water and organic solvents
| Composition | Al2O3SiO2 - xH2O |
|---|---|
| Density | about 2.5 g/ml |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 174
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "Clay." Accessed 11 Sep, 2003 .
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.8-2.6