Difference between revisions of "Sodium chlorate"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
White, water-soluble crystals. Sodium chlorate is a strong bleaching and oxidizing agent. It is used in bleaching paper pulps and was occasionally used for removing stains from archival papers (Roberts and Etherington 1982). However, it can leave residual chloride that is difficult to remove. Sodium chlorate is also used to dye and print textiles and to tan and finish leathers. | White, water-soluble crystals. Sodium chlorate is a strong bleaching and oxidizing agent. It is used in bleaching paper pulps and was occasionally used for removing stains from archival papers (Roberts and Etherington 1982). However, it can leave residual chloride that is difficult to remove. Sodium chlorate is also used to dye and print textiles and to tan and finish leathers. | ||
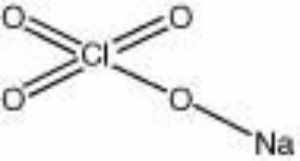
| + | [[[SliderGallery rightalign|sodium chlorate.jpg~Chemical structure]]] | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Fire hazard in contact with dry organic materials. | ||
| + | * Contact may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=S25540&productDescription=SODIUM+CHLORATE+500G+LAB+GRN&vendorId=VN00115888&countryCode=US&language=en SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water, glycerol. Insoluble in ethanol. | Soluble in water, glycerol. Insoluble in ethanol. | ||
| Line 18: | Line 23: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 248-260 | + | | 248-260 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.490 | + | | 2.490 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| mol. wt. = 106.5 | | mol. wt. = 106.5 | ||
| − | |||
| − | |||
| − | |||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 686 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
* ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | * ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | ||
| Line 52: | Line 44: | ||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8741 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8741 | ||
Latest revision as of 14:44, 1 June 2022
Description
White, water-soluble crystals. Sodium chlorate is a strong bleaching and oxidizing agent. It is used in bleaching paper pulps and was occasionally used for removing stains from archival papers (Roberts and Etherington 1982). However, it can leave residual chloride that is difficult to remove. Sodium chlorate is also used to dye and print textiles and to tan and finish leathers.
Risks
- Fire hazard in contact with dry organic materials.
- Contact may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, glycerol. Insoluble in ethanol.
| Composition | NaClO3 |
|---|---|
| CAS | 7775-09-9 |
| Melting Point | 248-260 C |
| Density | 2.490 g/ml |
| Molecular Weight | mol. wt. = 106.5 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 686
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8741
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998