Difference between revisions of "Sodium dithionite"
Jump to navigation
Jump to search
| (One intermediate revision by one other user not shown) | |||
| Line 4: | Line 4: | ||
Note: this is not the same compound as [[sodium%20thiosulfate|sodium thiosulfate]] (Na2S2O3; also called sodium hyposulfite) that is used for fixation in photography. | Note: this is not the same compound as [[sodium%20thiosulfate|sodium thiosulfate]] (Na2S2O3; also called sodium hyposulfite) that is used for fixation in photography. | ||
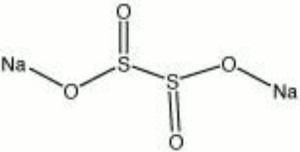
| − | + | [[[SliderGallery rightalign|sodium dithionite.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
sodium thiosulfite; sodium hydrosulfite; sodium sulfoxylate | sodium thiosulfite; sodium hydrosulfite; sodium sulfoxylate | ||
| − | |||
| − | == | + | |
| + | == Physical and Chemical Properties == | ||
Soluble in water (pH = 6.0-7.5 for 1-6% solution). Insoluble in ethanol. | Soluble in water (pH = 6.0-7.5 for 1-6% solution). Insoluble in ethanol. | ||
| Line 24: | Line 24: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 52-55 | + | | 52-55 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.19 | + | | 2.19 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 33: | ||
|} | |} | ||
| − | == | + | == Risks == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Fire risk in contact with moisture and air. Use dry sand to extinguish fires. Flash point=90 C | |
| + | * Contact causes irritation | ||
| + | * Integra Chemc: [https://www.integrachem.com/msds/S365_26390_102.pdf SDS] | ||
| − | == | + | ==Resources and Citations== |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 786 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 786 | ||
| Line 61: | Line 55: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8771 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8771 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Sodium_dithionite (Accessed Jan. 6 2006) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 16:35, 1 June 2022
Description
Pale yellow powder that is used as a Reducing agent in dyeing Indigo and vat dyes. Sodium dithionite is also used to strip dyes from dyed textiles and reduce iron oxide stains to Ferrous oxide. Sodium dithionite was also used as a bleach for Leather and mechanical paper pulps but its use has declined in recent years due to poor color reversion properties (AIC Book and Paper Catalog).
Note: this is not the same compound as Sodium thiosulfate (Na2S2O3; also called sodium hyposulfite) that is used for fixation in photography.
Synonyms and Related Terms
sodium thiosulfite; sodium hydrosulfite; sodium sulfoxylate
Physical and Chemical Properties
Soluble in water (pH = 6.0-7.5 for 1-6% solution). Insoluble in ethanol.
| Composition | Na2S2O4 |
|---|---|
| CAS | 7775-14-6 |
| Melting Point | 52-55 C |
| Density | 2.19 g/ml |
| Molecular Weight | mol. wt. = 174.1 |
Risks
- Fire risk in contact with moisture and air. Use dry sand to extinguish fires. Flash point=90 C
- Contact causes irritation
- Integra Chemc: SDS
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 786
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8771
- Wikipedia: http://en.wikipedia.org/wiki/Sodium_dithionite (Accessed Jan. 6 2006)