Difference between revisions of "Succinic acid"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 9: | Line 9: | ||
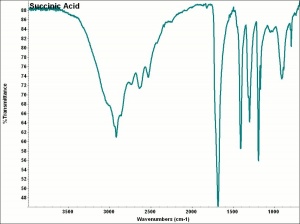
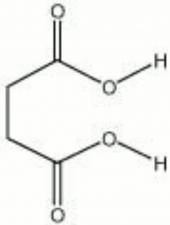
[[[SliderGallery rightalign|aaiSUCCINIC.jpg~FTIR|succinic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|aaiSUCCINIC.jpg~FTIR|succinic acid.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Contact causes irritation and possible burns. | ||
| + | * Fisher Scientific: [https://www.fishersci.com/store/msds?partNumber=S25790&productDescription=SUCCINIC+ACID+100+G&vendorId=VN00115888&countryCode=US&language=en SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in ethanol, ether. Slightly soluble in water: pH =2.7 (0.1 M solution) | Soluble in ethanol, ether. Slightly soluble in water: pH =2.7 (0.1 M solution) | ||
| Line 24: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 185-1.87 | + | | 185-1.87 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.55-1.56 | + | | 1.55-1.56 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 235 | + | | 235 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 54 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 54 | ||
Latest revision as of 12:29, 6 June 2022
Description
Colorless, odorless crystals that occur naturally in Amber. Succinic acid was first separated from the distillate of amber in 1546 by Agricola. Commercially, succinic acid is used as a Sequestrant and Buffer. It is also used in the manufacture of dyes, lacquers, and photographic solutions.
Synonyms and Related Terms
butanedioic acid; acid of amber; amber acid; ethylenesuccinic acid; BernsteinsSure (Deut.)
Risks
- Contact causes irritation and possible burns.
- Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in ethanol, ether. Slightly soluble in water: pH =2.7 (0.1 M solution)
Insoluble in benzene, carbon disulfide, carbon tetrachloride, ligroin.
| Composition | CO2H(CH2)2CO2H |
|---|---|
| CAS | 110-15-6 |
| Melting Point | 185-1.87 C |
| Density | 1.55-1.56 g/ml |
| Molecular Weight | mol. wt. = 118.1 |
| Boiling Point | 235 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 54
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9037
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985