Difference between revisions of "Sulfuric acid"
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | An oily, fuming, corrosive liquid. Sulfuric acid was the highest volume chemical produced in the USA in 1991. Valentine, a 15th century German alchemist , is credited with its discovery (Schur 1985). Called oil of vitriol, it was prepared by the distillation green vitriol ([ | + | An oily, fuming, corrosive liquid. Sulfuric acid was the highest volume chemical produced in the USA in 1991. Valentine, a 15th century German alchemist , is credited with its discovery (Schur 1985). Called oil of vitriol, it was prepared by the distillation green vitriol ([[ferrous%20sulfate|ferrous sulfate]]) and blue vitriol ([[copper%20sulfate|copper sulfate]]). Sulfuric acid is very reactive, dissolving most metals and sulfonating most organic compounds. It is used in etching and cleaning solutions for printing and photography. Sulfuric acid is also used in leather manufacture for bleaching, deliming, and pickling. Oleum or Nordhausen acid are common names used for fuming sulfuric acid (93-96%). Brown oil of vitriol (B.O.V.) is an old name for concentrated sulfuric acid (about 70%). |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
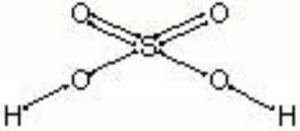
[[[SliderGallery rightalign|sulfuric acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|sulfuric acid.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| − | + | * Reacts exothermically with water (can be explosive). | |
| + | * Highly corrosive. | ||
| + | * Contact will destroy tissue. | ||
| + | * Highly toxic by ingestion and inhalation due to destruction of tissue. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC424525000&productDescription=SULFURIC+ACID+ACS+500ML&vendorId=VN00033901&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
| − | pH of concentrated solution=0.3; pH of 0.1N solution=1.2, pH of 0.01N solution=2.1 | + | * Miscible with water (add acid slowly to water). |
| + | * pH of concentrated solution=0.3; pH of 0.1N solution=1.2, pH of 0.01N solution=2.1 | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 10.4 | + | | 10.4 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.84 (pure) | + | | 1.84 g/ml (pure) |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 36: | Line 42: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 315-338 | + | | 315-338 C |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 785 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 785 | ||
| Line 65: | Line 61: | ||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * | + | * Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index =1.427; pH of concentrated solution=0.3; pH of 0.1N solution=1.2, pH of 0.01N solution=2.1 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index =1.427; pH of concentrated solution=0.3; pH of 0.1N solution=1.2, pH of 0.01N solution=2.1 | ||
Latest revision as of 09:20, 7 June 2022
Description
An oily, fuming, corrosive liquid. Sulfuric acid was the highest volume chemical produced in the USA in 1991. Valentine, a 15th century German alchemist , is credited with its discovery (Schur 1985). Called oil of vitriol, it was prepared by the distillation green vitriol (Ferrous sulfate) and blue vitriol (Copper sulfate). Sulfuric acid is very reactive, dissolving most metals and sulfonating most organic compounds. It is used in etching and cleaning solutions for printing and photography. Sulfuric acid is also used in leather manufacture for bleaching, deliming, and pickling. Oleum or Nordhausen acid are common names used for fuming sulfuric acid (93-96%). Brown oil of vitriol (B.O.V.) is an old name for concentrated sulfuric acid (about 70%).
Synonyms and Related Terms
hydrogen sulfate; battery acid; electrolyte acid; sulphuric acid (Br.), oil of vitriol; BOV; brown oil of vitriol; oleum; Nordhausen acid
Risks
- Reacts exothermically with water (can be explosive).
- Highly corrosive.
- Contact will destroy tissue.
- Highly toxic by ingestion and inhalation due to destruction of tissue.
- ThermoFisher: SDS
Physical and Chemical Properties
- Miscible with water (add acid slowly to water).
- pH of concentrated solution=0.3; pH of 0.1N solution=1.2, pH of 0.01N solution=2.1
| Composition | H2SO4 |
|---|---|
| CAS | 7664-93-9 |
| Melting Point | 10.4 C |
| Density | 1.84 g/ml (pure) |
| Molecular Weight | mol. wt. = 98.1 |
| Refractive Index | 1.427 |
| Boiling Point | 315-338 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 785
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index =1.427; pH of concentrated solution=0.3; pH of 0.1N solution=1.2, pH of 0.01N solution=2.1