Difference between revisions of "Tartaric acid"
Jump to navigation
Jump to search
| Line 2: | Line 2: | ||
Colorless, transparent crystals that occur naturally in wine lees. Tartaric acid is used in baking powders (potassium hydrogen tartrate), leather tanning and effervescent beverages. Tartaric acid acts as a [[buffer|buffering agent]] and [[sequestrant|sequestrant]]. It is used by dyers to print a blue ferric tartrate color and to remove some mordants from solution. Tartaric acid (and tartrates) reacts with ammonio-silver nitrate to produce metallic [[silver|silver]]; this reaction is used in photographic developing solutions and for silvering mirrors. | Colorless, transparent crystals that occur naturally in wine lees. Tartaric acid is used in baking powders (potassium hydrogen tartrate), leather tanning and effervescent beverages. Tartaric acid acts as a [[buffer|buffering agent]] and [[sequestrant|sequestrant]]. It is used by dyers to print a blue ferric tartrate color and to remove some mordants from solution. Tartaric acid (and tartrates) reacts with ammonio-silver nitrate to produce metallic [[silver|silver]]; this reaction is used in photographic developing solutions and for silvering mirrors. | ||
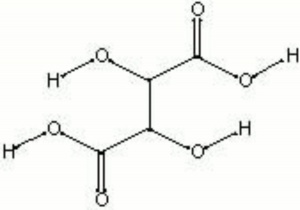
| − | + | [[[SliderGallery rightalign|tartaric acid.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
tartar; dihydroxysuccinic acid; L-tartaric acid; racemic acid; uvic acid; Weinsäure (Deut.); Ácido tartárico (Esp., Port.); Wijnsteenzuur (Ned.) | tartar; dihydroxysuccinic acid; L-tartaric acid; racemic acid; uvic acid; Weinsäure (Deut.); Ácido tartárico (Esp., Port.); Wijnsteenzuur (Ned.) | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Combustible. Flash point = 210 C | ||
| + | * Corrosive. | ||
| + | * Contact cause irritation and burns. | ||
| + | * Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-t/S25601A.pdf SDS] | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 32: | Line 37: | ||
| mol. wt. = 150.1 | | mol. wt. = 150.1 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Resources and Citations== | ==Resources and Citations== | ||
Latest revision as of 08:56, 8 June 2022
Description
Colorless, transparent crystals that occur naturally in wine lees. Tartaric acid is used in baking powders (potassium hydrogen tartrate), leather tanning and effervescent beverages. Tartaric acid acts as a buffering agent and Sequestrant. It is used by dyers to print a blue ferric tartrate color and to remove some mordants from solution. Tartaric acid (and tartrates) reacts with ammonio-silver nitrate to produce metallic Silver; this reaction is used in photographic developing solutions and for silvering mirrors.
Synonyms and Related Terms
tartar; dihydroxysuccinic acid; L-tartaric acid; racemic acid; uvic acid; Weinsäure (Deut.); Ácido tartárico (Esp., Port.); Wijnsteenzuur (Ned.)
Risks
- Combustible. Flash point = 210 C
- Corrosive.
- Contact cause irritation and burns.
- Fisher Scientific: SDS
Physical and Chemical Properties
- Soluble in water, ethanol, ether, glycerol. Insoluble in chloroform.
- Smells of burnt sugar when heated.
- pH = 2.2 (0.1 N solution)
| Composition | HOOC(CH2O)2COOH |
|---|---|
| CAS | 133-37-9 |
| Melting Point | 170 C |
| Density | 1.76 g/ml |
| Molecular Weight | mol. wt. = 150.1 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 68
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9235
- Wikipedia: http://en.wikipedia.org/wiki/Tartaric_acid (Accessed Sept. 28, 2005)
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH = 2.2 (0.1 N solution)
- Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm