Difference between revisions of "Titanium trichloride"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
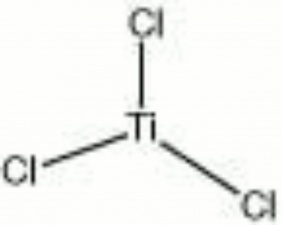
== Description == | == Description == | ||
| − | + | [[[SliderGallery rightalign|titanium trichloride.jpg~Chemical structure]]] | |
Dark violet, unstable, deliquescent crystals. Titanium trichloride is a very strong reducing agent that is used for stripping dyes and bleaching stains. | Dark violet, unstable, deliquescent crystals. Titanium trichloride is a very strong reducing agent that is used for stripping dyes and bleaching stains. | ||
| Line 7: | Line 7: | ||
titanous chloride; titanium (III) chloride | titanous chloride; titanium (III) chloride | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Fire risk in the presence of organic compounds. | ||
| + | * Decomposes in moist air with substantial generation of heat. | ||
| + | * Corrosive. | ||
| + | * Skin contact causes irritation and burns. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/96806.htm MSDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in ethanol, acetonitrile, water (generates heat). Slightly soluble in chloroform. Insoluble in ether and hydrocarbons. | Soluble in ethanol, acetonitrile, water (generates heat). Slightly soluble in chloroform. Insoluble in ether and hydrocarbons. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 440 (dec) | + | | 440 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| Line 31: | Line 37: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 45: | Line 45: | ||
* Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | ||
| − | * Website | + | * Website: www.hants.org.uk/museums/ofr/cmeth_t.html |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:26, 10 June 2022
Description
Dark violet, unstable, deliquescent crystals. Titanium trichloride is a very strong reducing agent that is used for stripping dyes and bleaching stains.
Synonyms and Related Terms
titanous chloride; titanium (III) chloride
Risks
- Fire risk in the presence of organic compounds.
- Decomposes in moist air with substantial generation of heat.
- Corrosive.
- Skin contact causes irritation and burns.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ethanol, acetonitrile, water (generates heat). Slightly soluble in chloroform. Insoluble in ether and hydrocarbons.
| Composition | TiCl3 |
|---|---|
| CAS | 7705-07-9 |
| Melting Point | 440 C (dec) |
| Density | 2.6 |
| Molecular Weight | mol. wt. = 154.23 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9620
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Website: www.hants.org.uk/museums/ofr/cmeth_t.html