Difference between revisions of "Trichloroacetic acid"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White, deliquescent crystals precipitate [ | + | White, deliquescent crystals precipitate [[protein|proteins]] and is used as a reagent for the detection of [[albumin|albumin]]. Trichloroacetic acid is used in the manufacture of pharmaceuticals and herbicides. |
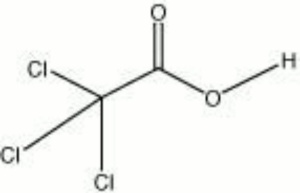
| − | + | [[[SliderGallery rightalign|trichloroacetic acid.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
TCA | TCA | ||
| − | + | == Risks == | |
| − | == | + | * Toxic by ingestion and inhalation. |
| + | * Highly corrosive on contact. | ||
| + | * Decomposes to form chloroform, hydrochloric acid, carbon dioxide, carbon monoxide. | ||
| + | * ThermoFisher: [https://www.fishersci.com/msdsproxy%3FproductName%3DBP5551%26productDescription%3DTRICHLOROACETIC%2BACID%2B1KG%26catNo%3DBP555-1%26vendorId%3DVN00033897%26storeId%3D10652 SDS] | ||
| + | ==Physical and Chemical Properties== | ||
| − | Soluble in water, ethanol, ether. pH = 1.2 (for 0.1 M solution). | + | * Soluble in water, ethanol, ether. |
| + | * pH = 1.2 (for 0.1 M solution). | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 57-58 | + | | 57-58 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.6298 | + | | 1.6298 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 36: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 196-197 | + | | 196-197 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 11:52, 16 June 2022
Description
White, deliquescent crystals precipitate proteins and is used as a reagent for the detection of Albumin. Trichloroacetic acid is used in the manufacture of pharmaceuticals and herbicides.
Synonyms and Related Terms
TCA
Risks
- Toxic by ingestion and inhalation.
- Highly corrosive on contact.
- Decomposes to form chloroform, hydrochloric acid, carbon dioxide, carbon monoxide.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in water, ethanol, ether.
- pH = 1.2 (for 0.1 M solution).
| Composition | CCl3COOH |
|---|---|
| CAS | 76-03-9 |
| Melting Point | 57-58 C |
| Density | 1.6298 g/ml |
| Molecular Weight | mol. wt. = 163.39 |
| Boiling Point | 196-197 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9756