Difference between revisions of "Vandyke brown"
(username removed) |
|||
| (9 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | Vandyke Brown, also known as [ | + | Vandyke Brown, also known as [[Cassel%20brown|Cassel earth]], is a brown, organic [[humus|humus]], [[peat|peat]], or [[coal|coal]] that contains iron oxides. It was first used in the 17th century and is still in use. Early sources for Vandyke brown were from the Cologne and Kassel regions of Germany with the regional source providing the pigment name. The brown earth has since been obtained from various localities each of which may differ slightly in color and composition. To add to this confusion some pigments labeled Vandyke brown were bituminous while others were synthetically made from from [[carbon%20black|carbon black]] and [[iron%20oxide%20red|iron oxide]] mixtures. When natural Vandyke brown is ignited, the pigment leaves a soft gray residue. It is not soluble in petroleum solvents. The colorant has poor lightfastness and fades on exposure to [[ultraviolet%20radiation|UV radiation]]. |
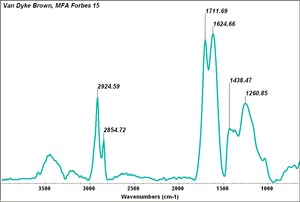
| + | [[[SliderGallery rightalign|Van Dyke Brown, MFA Forbes 15.TIF~FTIR (MFA)]]] | ||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | van Dyke Brown; Natural Brown 8; CI 77727; terre de Cologne (Fr.); terre de Cassel (Fr.); brun van Dyck (Fr.); terra di Colonia (It.);terra di Cassel (It.); Van Dyck Braun (Deut.); Kasslerbraun (Deut.); Kasseler Erde (Deut.); | + | van Dyke Brown; Natural Brown 8; CI 77727; terre de Cologne (Fr.); terre de Cassel (Fr.); brun van Dyck (Fr.); terra di Colonia (It.);terra di Cassel (It.); Van Dyck Braun (Deut.); Kasslerbraun (Deut.); Kasseler Erde (Deut.); Kölnische Erde (Deut.); tierra de Cassel (Esp.); pardo Van Dyck (Esp.); Cassel earth; Cassel's earth; cologne earth; Cassel brown; Kassel earth; ulmin brown; bruno Vandyck; Ruben's brown; coal brown; sap brown dye |
| − | == | + | == Risks == |
| − | + | * No significant hazards. | |
| + | == Physical and Chemical Properties == | ||
| − | Burns producing a tarry smoke and gray residue. | + | * Irregular particle shape with little, if any, birefringence |
| + | * Burns producing a tarry smoke and gray residue. | ||
| + | * Soluble in alkalis. | ||
| + | * Fades in sunlight. | ||
| + | * Density = 1.66 | ||
| + | * Refractive Index = 1.62-1.69 | ||
| − | + | == Resources and Citations == | |
| − | + | * R.Feller, R.Johnston-Feller, "Vandyke Brown", ''Artists Pigments'', Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. | |
| − | + | * Georgiana Languri, Molecular studies of Asphalt, Mummy and Kassel earth pigments, MOLART report 2004, available through Archetype Publications, London. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Helmut Schweppe, Schweppe color collection index and information book | |
| − | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 452 | |
| − | + | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | |
| − | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | |
| − | + | * Tom Rowland, Noel Riley, ''A-Z Guide to Cleaning, Conserving and Repairing Antiques'', Constable and Co., Ltd., London, 1981 | |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | * | + | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * Website | + | * Website: www.handprint.com |
| − | * | + | * Pigments through the ages - http://webexhibits.org/pigments/indiv/overview/vandyke.html |
* Colour Index International online at www.colour-index.org | * Colour Index International online at www.colour-index.org | ||
Latest revision as of 13:22, 23 June 2022
Description
Vandyke Brown, also known as Cassel earth, is a brown, organic Humus, Peat, or Coal that contains iron oxides. It was first used in the 17th century and is still in use. Early sources for Vandyke brown were from the Cologne and Kassel regions of Germany with the regional source providing the pigment name. The brown earth has since been obtained from various localities each of which may differ slightly in color and composition. To add to this confusion some pigments labeled Vandyke brown were bituminous while others were synthetically made from from Carbon black and iron oxide mixtures. When natural Vandyke brown is ignited, the pigment leaves a soft gray residue. It is not soluble in petroleum solvents. The colorant has poor lightfastness and fades on exposure to UV radiation.
Synonyms and Related Terms
van Dyke Brown; Natural Brown 8; CI 77727; terre de Cologne (Fr.); terre de Cassel (Fr.); brun van Dyck (Fr.); terra di Colonia (It.);terra di Cassel (It.); Van Dyck Braun (Deut.); Kasslerbraun (Deut.); Kasseler Erde (Deut.); Kölnische Erde (Deut.); tierra de Cassel (Esp.); pardo Van Dyck (Esp.); Cassel earth; Cassel's earth; cologne earth; Cassel brown; Kassel earth; ulmin brown; bruno Vandyck; Ruben's brown; coal brown; sap brown dye
Risks
- No significant hazards.
Physical and Chemical Properties
- Irregular particle shape with little, if any, birefringence
- Burns producing a tarry smoke and gray residue.
- Soluble in alkalis.
- Fades in sunlight.
- Density = 1.66
- Refractive Index = 1.62-1.69
Resources and Citations
- R.Feller, R.Johnston-Feller, "Vandyke Brown", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.
- Georgiana Languri, Molecular studies of Asphalt, Mummy and Kassel earth pigments, MOLART report 2004, available through Archetype Publications, London.
- Helmut Schweppe, Schweppe color collection index and information book
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 452
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website: www.handprint.com
- Pigments through the ages - http://webexhibits.org/pigments/indiv/overview/vandyke.html
- Colour Index International online at www.colour-index.org