Difference between revisions of "Potassium bicarbonate"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Colorless crystals or white powder. Potassium bicarbonate is used to add [ | + | Colorless crystals or white powder. Potassium bicarbonate is used to add [[carbon%20dioxide|carbon dioxide]] to water, as in soft drinks. It is also used in baking powders, [[fire%20extinguisher|fire extinguishers]], and to adjust the [[pH|pH]] of [[detergent|detergents]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
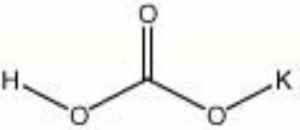
[[[SliderGallery rightalign|potassium bicarbonate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|potassium bicarbonate.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/01245.htm MSDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in water, pH = 8.2 (0.1 M solution). Insoluble in ethanol. Slightly alkaline. | Soluble in water, pH = 8.2 (0.1 M solution). Insoluble in ethanol. Slightly alkaline. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 100-120 (dec) | + | | 100-120 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.17 | + | | 2.17 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 38: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7770 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7770 | ||
Latest revision as of 07:09, 26 July 2022
Description
Colorless crystals or white powder. Potassium bicarbonate is used to add Carbon dioxide to water, as in soft drinks. It is also used in baking powders, fire extinguishers, and to adjust the PH of detergents.
Synonyms and Related Terms
potassium acid carbonate; baking soda
Risks
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water, pH = 8.2 (0.1 M solution). Insoluble in ethanol. Slightly alkaline.
| Composition | KHCO3 |
|---|---|
| CAS | 298-14-6 |
| Melting Point | 100-120 C (dec) |
| Density | 2.17 g/ml |
| Molecular Weight | mol. wt. = 100.1 |
| Refractive Index | 1.380, 1.482, 1.578 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7770
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.380, 1.482, 1.578