Difference between revisions of "Quinacrine dihydrochloride"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
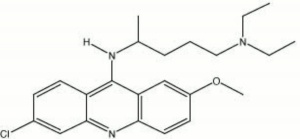
| − | A bright yellow [ | + | A bright yellow [[fluorochrome|fluorochrome]] powder. Quinacrine dihydrochloride dissolves in hot water to produce a solution that is vividly fluorescent under [[ultraviolet%20radiation|ultraviolet light]]. The mean excitation wavelength for quinacrine dihydrochloride is 440 nm and the mean emission wavelength is 510 nm (Wolbers, et al, 1990). |
| − | + | [[[SliderGallery rightalign|quinacrine dihydrochloride.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
quinacrine mustard; quinacrine dihydrochloride dihydrate; Atabrine hydrochloride; RP-866; SN-390; mepacrine | quinacrine mustard; quinacrine dihydrochloride dihydrate; Atabrine hydrochloride; RP-866; SN-390; mepacrine | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Toxic. | ||
| + | * Light sensitive. | ||
| + | * Hygroscopic. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/36669.htm MSDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in hot water (pH = 4.5 for 1% solution). Slightly soluble in cold water, ethanol and methanol. Insoluble in ether, benzene, acetone. | Soluble in hot water (pH = 4.5 for 1% solution). Slightly soluble in cold water, ethanol and methanol. Insoluble in ether, benzene, acetone. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 250 (dec) | + | | 250 C (dec) |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 33: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 199 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 199 | ||
Latest revision as of 12:59, 27 September 2022
Description
A bright yellow Fluorochrome powder. Quinacrine dihydrochloride dissolves in hot water to produce a solution that is vividly fluorescent under ultraviolet light. The mean excitation wavelength for quinacrine dihydrochloride is 440 nm and the mean emission wavelength is 510 nm (Wolbers, et al, 1990).
Synonyms and Related Terms
quinacrine mustard; quinacrine dihydrochloride dihydrate; Atabrine hydrochloride; RP-866; SN-390; mepacrine
Risks
- Toxic.
- Light sensitive.
- Hygroscopic.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in hot water (pH = 4.5 for 1% solution). Slightly soluble in cold water, ethanol and methanol. Insoluble in ether, benzene, acetone.
| Composition | C23H30ClN3O-2HCl-2H2O |
|---|---|
| CAS | 69-05-6 |
| Melting Point | 250 C (dec) |
| Molecular Weight | mol. wt. = 508.6293 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 199
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8225
- Aldrich Chemical Catalog