Difference between revisions of "Methyl orange, sodium salt"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A pale, orange powder used as an acid-base [ | + | A pale, orange powder used as an acid-base |
| − | + | [[indicator%20dye|indicator]]. Methyl orange is an azo dye that was discovered by several workers near the same time period: P. Griess in 1875; O.N. Witt in 1876; and Z. Roussin in 1876. As an indicator, methyl orange forms a red color below pH 3.1, turns orange above pH 4.4 and becomes yellow in alkaline solutions. It is used for titrating | |
| + | [[inorganic%20acid|mineral acids]]. Methyl orange is also used for dyeing | ||
| + | [[textile|textiles]]. | ||
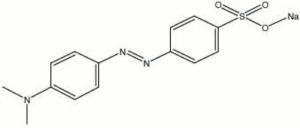
| + | [[[SliderGallery rightalign|methyl orange, sodium salt.jpg~Chemical structure]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
4-[[(4-dimethylamino)phenyl]-azo]benzenesulfonic acid sodium salt; sodium p-dimethylaminoazobenzenesulfonate; helianthine B; Acid Orange 52; CI 13025; Orange III; Gold Orange; Topaeolin D | 4-[[(4-dimethylamino)phenyl]-azo]benzenesulfonic acid sodium salt; sodium p-dimethylaminoazobenzenesulfonate; helianthine B; Acid Orange 52; CI 13025; Orange III; Gold Orange; Topaeolin D | ||
| − | + | == Risks == | |
| − | == | + | * Toxic by ingestion. |
| + | * Inhalation and skin contact may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=M21625&productDescription=METHYL+ORANGE+CERT+ACS+25G&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Soluble in hot water. Insoluble in ethanol. | Soluble in hot water. Insoluble in ethanol. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.00 | + | | 1.00 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 34: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 6180 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 6180 | ||
Latest revision as of 13:22, 1 October 2022
Description
A pale, orange powder used as an acid-base
indicator. Methyl orange is an azo dye that was discovered by several workers near the same time period: P. Griess in 1875; O.N. Witt in 1876; and Z. Roussin in 1876. As an indicator, methyl orange forms a red color below pH 3.1, turns orange above pH 4.4 and becomes yellow in alkaline solutions. It is used for titrating
mineral acids. Methyl orange is also used for dyeing
textiles.
Synonyms and Related Terms
4-[[(4-dimethylamino)phenyl]-azo]benzenesulfonic acid sodium salt; sodium p-dimethylaminoazobenzenesulfonate; helianthine B; Acid Orange 52; CI 13025; Orange III; Gold Orange; Topaeolin D
Risks
- Toxic by ingestion.
- Inhalation and skin contact may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in hot water. Insoluble in ethanol.
| Composition | (CH3)2NC6H4NNC6H4SO3Na |
|---|---|
| CAS | 547-58-0 |
| Density | 1.00 g/ml |
| Molecular Weight | mol. wt.=327.34 |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 6180
- Colour Index International online at www.colour-index.org
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993