Difference between revisions of "Microcrystalline wax"
(username removed) |
|||
| (8 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A high molecular weight hydrocarbon wax that has a fine crystalline structure. Microcrystalline waxes were first made in the late 1930s by Baker Petrolite in Barnsdall, Oklahoma. It is the remaining fraction of [ | + | A high molecular weight hydrocarbon wax that has a fine crystalline structure. Microcrystalline waxes were first made in the late 1930s by Baker Petrolite in Barnsdall, Oklahoma. It is the remaining fraction of [[paraffin wax]] after the lower molecular weight waxes are removed. Microcrystalline wax is chemically inert, and in general, a stronger adhesive than paraffin wax. It can be softened by adding [[mineral oil]] or [[petroleum solvent]]. Microcrystalline wax does not emulsify easily but can be modified with a catalyst to produce an oxidized, emulsifiable form that is used in hard, self-polishing floor wax. Microcrystalline wax is used in laminating paper and foils as well as for polishes. It polishes to a glass clear, smooth, non-sticky finish. |
| − | |||
| − | |||
| + | See also [[mineral wax]]. | ||
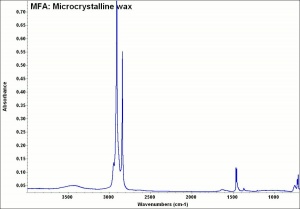
| + | [[[SliderGallery rightalign|MFA- Microcrystalline wax.jpg~FTIR]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
petrolatum wax; cera microcristalina (Esp.); cire microcristalline (Fr.); cera microcristallina (It); | petrolatum wax; cera microcristalina (Esp.); cire microcristalline (Fr.); cera microcristallina (It); | ||
| + | Examples include: Multiwax W445; Bareco® 85C (185F); Be Square® 77-92C (170-197F); Bareco® Victory 74C (165F); Cosmolloid 85C (185F); Renaissance Wax | ||
| − | |||
| − | |||
| − | |||
| − | == | + | == Physical and Chemical Properties == |
Iodine value=0, acid value=0, saponification value=0 | Iodine value=0, acid value=0, saponification value=0 | ||
| Line 20: | Line 18: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 60-93 | + | | 60-93 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.915-0.941 | + | | 0.915-0.941 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 33: | Line 31: | ||
[[media:download_file_20.pdf|Properties of Natural Waxes]] | [[media:download_file_20.pdf|Properties of Natural Waxes]] | ||
| − | + | == Resources and Citations == | |
| − | |||
| − | == | ||
* ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | * ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 591 |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * John S. Mills, Raymond White, ''The Organic Chemistry of Museum Objects'', Butterworth Heineman, London, 2nd ed., 1994 |
* ''A History of Technology'', Charles Singer, E.J. Holmyard, A.R. Hall (eds.), Clarendon Press, Oxford, Volume 1: From Early times to Fall of Ancient Empires, 1954 | * ''A History of Technology'', Charles Singer, E.J. Holmyard, A.R. Hall (eds.), Clarendon Press, Oxford, Volume 1: From Early times to Fall of Ancient Empires, 1954 | ||
| Line 49: | Line 45: | ||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point=71-89C, density=0.928-0.941, ref. index = 1.441, iodine value=0, acid value=0, saponification value=0 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point=71-89C, density=0.928-0.941, ref. index = 1.441, iodine value=0, acid value=0, saponification value=0 | ||
| − | * | + | * Tom Rowland, Noel Riley, ''A-Z Guide to Cleaning, Conserving and Repairing Antiques'', Constable and Co., Ltd., London, 1981 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Microcrystalline_wax (Accessed Feb. 10, 2006) |
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:21, 1 October 2022
Description
A high molecular weight hydrocarbon wax that has a fine crystalline structure. Microcrystalline waxes were first made in the late 1930s by Baker Petrolite in Barnsdall, Oklahoma. It is the remaining fraction of Paraffin wax after the lower molecular weight waxes are removed. Microcrystalline wax is chemically inert, and in general, a stronger adhesive than paraffin wax. It can be softened by adding Mineral oil or Petroleum solvent. Microcrystalline wax does not emulsify easily but can be modified with a catalyst to produce an oxidized, emulsifiable form that is used in hard, self-polishing floor wax. Microcrystalline wax is used in laminating paper and foils as well as for polishes. It polishes to a glass clear, smooth, non-sticky finish.
See also Mineral wax.
Synonyms and Related Terms
petrolatum wax; cera microcristalina (Esp.); cire microcristalline (Fr.); cera microcristallina (It); Examples include: Multiwax W445; Bareco® 85C (185F); Be Square® 77-92C (170-197F); Bareco® Victory 74C (165F); Cosmolloid 85C (185F); Renaissance Wax
Physical and Chemical Properties
Iodine value=0, acid value=0, saponification value=0
| Melting Point | 60-93 C |
|---|---|
| Density | 0.915-0.941 g/ml |
| Refractive Index | 1.441 |
Comparisons
Resources and Citations
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 591
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- A History of Technology, Charles Singer, E.J. Holmyard, A.R. Hall (eds.), Clarendon Press, Oxford, Volume 1: From Early times to Fall of Ancient Empires, 1954
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point=71-89C, density=0.928-0.941, ref. index = 1.441, iodine value=0, acid value=0, saponification value=0
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Wikipedia: http://en.wikipedia.org/wiki/Microcrystalline_wax (Accessed Feb. 10, 2006)
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000