Difference between revisions of "Sodium bisulfate"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Colorless crystals or white lumps. Sodium bisulfate is used as an inexpensive substitute for [ | + | Colorless crystals or white lumps. Sodium bisulfate is used as an inexpensive substitute for [[sulfuric%20acid|sulfuric acid]] in dyeing solutions. It is also used in the manufacture of [[paper|paper]], [[soap|soap]], [[magnesia%20cement|magnesia cements]], and acid-based industrial cleaners. Sodium bisulfate acts as a [[flux|flux]] in metallurgy. It can pickle [[metal|metals]], carbonize [[wool|wool]], and bleach [[leather|leather]]. |
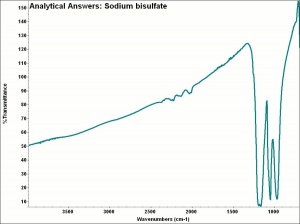
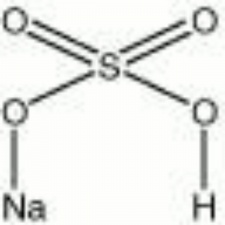
| − | + | [[[SliderGallery rightalign|aaiNAbiSO4.jpg~FTIR|sodium bisulfate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
sodium acid sulfate; niter cake; sodium hydrogen sulfate; sodium bisulphate (Br.); sodium pyrosulfate | sodium acid sulfate; niter cake; sodium hydrogen sulfate; sodium bisulphate (Br.); sodium pyrosulfate | ||
| − | + | == Risks == | |
| − | == | + | * Strongly irritating to skin, eyes and lungs. |
| + | * Hygroscopic. | ||
| + | * Noncombustible. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=S2403&productDescription=SODIUM+BISULFATE+CERTIFD+3KG&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Soluble in water forming an acidic solution (0.1 molar has a pH of 1.4). Decomposes in ethanol to form sodium sulfate and sulfuric acid. | Soluble in water forming an acidic solution (0.1 molar has a pH of 1.4). Decomposes in ethanol to form sodium sulfate and sulfuric acid. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 315 | + | | 315 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.435 | + | | 2.435 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 38: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8727 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8727 | ||
| − | * | + | * Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.43, 1.46, 1.47 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.43, 1.46, 1.47 | ||
| Line 54: | Line 52: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:20, 3 October 2022
Description
Colorless crystals or white lumps. Sodium bisulfate is used as an inexpensive substitute for Sulfuric acid in dyeing solutions. It is also used in the manufacture of Paper, Soap, magnesia cements, and acid-based industrial cleaners. Sodium bisulfate acts as a Flux in metallurgy. It can pickle metals, carbonize Wool, and bleach Leather.
Synonyms and Related Terms
sodium acid sulfate; niter cake; sodium hydrogen sulfate; sodium bisulphate (Br.); sodium pyrosulfate
Risks
- Strongly irritating to skin, eyes and lungs.
- Hygroscopic.
- Noncombustible.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water forming an acidic solution (0.1 molar has a pH of 1.4). Decomposes in ethanol to form sodium sulfate and sulfuric acid.
| Composition | NaHSO4 |
|---|---|
| CAS | 7681-38-1 |
| Melting Point | 315 C |
| Density | 2.435 g/ml |
| Molecular Weight | mol. wt. = 120.06 |
| Refractive Index | 1.43, 1.46, 1.47 |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8727
- Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.43, 1.46, 1.47
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998