Difference between revisions of "Magenta"
(username removed) |
|||
| (7 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:MFA504042 Magenta.jpg|thumb|Magenta dyed silk dress; MFA# 50.4042]] | ||
== Description == | == Description == | ||
| − | A synthetic aniline dyestuff that is also called fuchsin. Magenta was the second synthetic dye material to be produced from coal-tar derivatives. It was first noticed by Natanson in 1856. Then in 1859, Verguin patented a large scale production method for making magenta by oxidizing crude aniline with [ | + | A synthetic aniline dyestuff that is also called fuchsin. Magenta was the second synthetic dye material to be produced from coal-tar derivatives. It was first noticed by Natanson in 1856. Then in 1859, Verguin patented a large scale production method for making magenta by oxidizing crude aniline with [[stannic chloride]], a tanning solution. Magenta is a dark green water soluble powder that oxidizes to forms a deep red fugitive dye. It is used to color textiles and [[leather]] and to stain bacteria. Magenta was formerly used in watercolor paints but has since been replaced by colors with better lightfastness properties. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 8: | Line 9: | ||
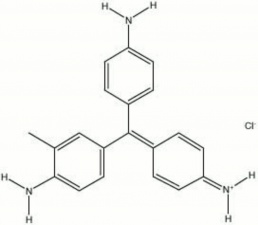
[[[SliderGallery rightalign|magenta.jpg~Chemical structure]]] | [[[SliderGallery rightalign|magenta.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| − | == | + | * Suspected carcinogen. |
| + | * Woodworker's Supply: [https://woodworker.com/pdf/a1930.pdf MSDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water, alcohol, acetone | Soluble in water, alcohol, acetone | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 250 | + | | 250 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.22 | + | | 1.22 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 35: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: Natanson =1856 | |
| − | + | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | |
| − | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | |
| − | * | + | * Website: http://www.smith.edu/hsc/silk/Papers/meredith.html (Natanson = 1856; Verguin = 1859) |
| − | * | + | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: Verguin patented process in 1859 |
| − | * | + | * F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 Comment: Natanson = 1856; Verguin = 1859 |
| − | * | + | * A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ''ICOM-CC Preprints'' Lyon, Getty Conservation Institute, Los Angeles, 1999 Comment: discovered 1856 |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Colour Index International online at www.colour-index.org | * Colour Index International online at www.colour-index.org | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:00, 16 October 2022
Description
A synthetic aniline dyestuff that is also called fuchsin. Magenta was the second synthetic dye material to be produced from coal-tar derivatives. It was first noticed by Natanson in 1856. Then in 1859, Verguin patented a large scale production method for making magenta by oxidizing crude aniline with Stannic chloride, a tanning solution. Magenta is a dark green water soluble powder that oxidizes to forms a deep red fugitive dye. It is used to color textiles and Leather and to stain bacteria. Magenta was formerly used in watercolor paints but has since been replaced by colors with better lightfastness properties.
Synonyms and Related Terms
rosaniline hydrochloride; CI Basic Red 9; CI 42510; Basic Violet 14; p-fuchsin; Solvent Red 41 (base); fuchsin; Magentarot (Deut.); magenta (Fr., Ned., Port.); fuschine (Fr.); fucsina (It.); basic fuchsin; fuchsine; azaleine (marketing name for magenta prepared by the Gerber Keller process); rosaniline chloride
Risks
- Suspected carcinogen.
- Woodworker's Supply: MSDS
Physical and Chemical Properties
Soluble in water, alcohol, acetone
| Composition | C20H20N3Cl |
|---|---|
| CAS | 632-99-5 |
| Melting Point | 250 C |
| Density | 1.22 g/ml |
| Molecular Weight | mol. wt. = 337.85 |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: Natanson =1856
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Website: http://www.smith.edu/hsc/silk/Papers/meredith.html (Natanson = 1856; Verguin = 1859)
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: Verguin patented process in 1859
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876 Comment: Natanson = 1856; Verguin = 1859
- A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ICOM-CC Preprints Lyon, Getty Conservation Institute, Los Angeles, 1999 Comment: discovered 1856
- Colour Index International online at www.colour-index.org
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000