Difference between revisions of "Mars colors"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | Originally a brand name in the late 18th century, Mars colors is now the common name used for synthetic iron oxide pigments. Mars colors are made from hydrated iron oxide co-precipitated with alum using [ | + | Originally a brand name in the late 18th century, Mars colors is now the common name used for synthetic iron oxide pigments. Mars colors are made from hydrated iron oxide co-precipitated with alum using [[lime|lime]] or [[potash|potash]]. The proportion of the mixture controls the degree of color. The product is dried to produce Mars yellow. Other colors, orange, red, black, brown, and violet result from calcining the yellow. Their chemical and physical properties are the same as the natural iron oxides. Mars colors are permanent pigments with good tinting strength and are good driers for oil paints. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Mars yellow; Mars orange; Mars violet; Mars brown; Mars black; Mars red; Mapico pigments; Mars pigments | Mars yellow; Mars orange; Mars violet; Mars brown; Mars black; Mars red; Mapico pigments; Mars pigments | ||
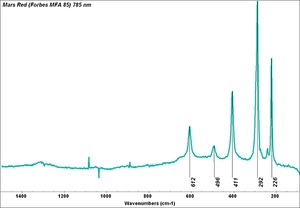
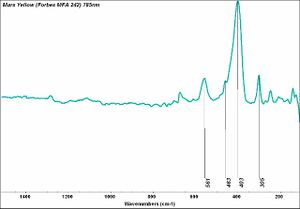
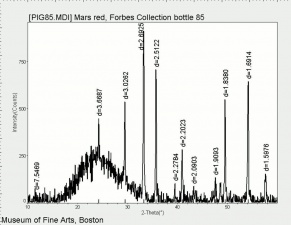
| + | [[[SliderGallery rightalign|Mars Red (Forbes MFA 85) 785 nm resize.tif~Raman Mars red (MFA)|Mars Yellow (Forbes MFA 242) 785nm (640x445).jpg~Raman yellow (MFA)|PIG85.jpg~XRD]]] | ||
| + | == Risks == | ||
| − | + | * No significant hazards. | |
| − | + | == Physical and Chemical Properties == | |
| − | == | ||
Lightfast. | Lightfast. | ||
| − | + | ==Resources and Citations== | |
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 30: | Line 26: | ||
* R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:06, 17 October 2022
Description
Originally a brand name in the late 18th century, Mars colors is now the common name used for synthetic iron oxide pigments. Mars colors are made from hydrated iron oxide co-precipitated with alum using Lime or Potash. The proportion of the mixture controls the degree of color. The product is dried to produce Mars yellow. Other colors, orange, red, black, brown, and violet result from calcining the yellow. Their chemical and physical properties are the same as the natural iron oxides. Mars colors are permanent pigments with good tinting strength and are good driers for oil paints.
Synonyms and Related Terms
Mars yellow; Mars orange; Mars violet; Mars brown; Mars black; Mars red; Mapico pigments; Mars pigments
Risks
- No significant hazards.
Physical and Chemical Properties
Lightfast.
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 319
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000