Difference between revisions of "Menthol"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white crystalline compound obtained from the distillation of [ | + | A white crystalline compound obtained from the distillation of [[peppermint%20oil|peppermint oil]]. Menthol was first isolated in 1771 by Gambius. In the late 19th century, menthol was used with [[naphthalene|naphthalene]] as a moth repellent. Now, it is used in perfumes, medicines, and cigarettes. |
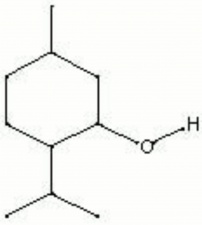
| − | + | [[[SliderGallery rightalign|menthol.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
hexahydrothymol; peppermint camphor; methylhydroxyisopropyl-cyclohexane; p-menthan-3-ol; mentol (Dan., Pol.); Menthol (Deut., Fr., Ned.); | hexahydrothymol; peppermint camphor; methylhydroxyisopropyl-cyclohexane; p-menthan-3-ol; mentol (Dan., Pol.); Menthol (Deut., Fr., Ned.); | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Combustible. Flash point = 93 C. | ||
| + | * Inhalation causes irritation to mucous membranes. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/16349.htm MSDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in ethanol, chloroform, ether, ligroin, glacial acetic acid. Slightly soluble in water. | Soluble in ethanol, chloroform, ether, ligroin, glacial acetic acid. Slightly soluble in water. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 41-43 | + | | 41-43 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.890 | + | | 0.890 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 35: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 212 | + | | 212 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * L. Goldberg, A History Of Pest Control Measures In The Anthropology Collections, National Museum Of Natural History, Smithsonian Institution, ''JAIC'' (35):23-43, 1996 | |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 586 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 586 | ||
| Line 50: | Line 46: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5882 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5882 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Menthol (Accessed Jan. 6, 2006) |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:23, 18 October 2022
Description
A white crystalline compound obtained from the distillation of Peppermint oil. Menthol was first isolated in 1771 by Gambius. In the late 19th century, menthol was used with Naphthalene as a moth repellent. Now, it is used in perfumes, medicines, and cigarettes.
Synonyms and Related Terms
hexahydrothymol; peppermint camphor; methylhydroxyisopropyl-cyclohexane; p-menthan-3-ol; mentol (Dan., Pol.); Menthol (Deut., Fr., Ned.);
Risks
- Combustible. Flash point = 93 C.
- Inhalation causes irritation to mucous membranes.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ethanol, chloroform, ether, ligroin, glacial acetic acid. Slightly soluble in water.
| Composition | CH3C6H9(C3H7)OH |
|---|---|
| CAS | 1490-04-6 (dl-menthol) |
| Melting Point | 41-43 C |
| Density | 0.890 g/ml |
| Molecular Weight | mol. wt. = 156.26 |
| Boiling Point | 212 C |
Resources and Citations
- L. Goldberg, A History Of Pest Control Measures In The Anthropology Collections, National Museum Of Natural History, Smithsonian Institution, JAIC (35):23-43, 1996
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 586
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5882
- Wikipedia: http://en.wikipedia.org/wiki/Menthol (Accessed Jan. 6, 2006)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998