Difference between revisions of "Naphthalene"
(username removed) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
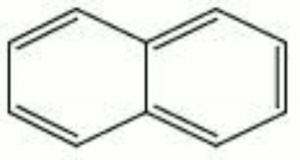
| − | White, crystalline flakes with a strong [ | + | White, crystalline flakes with a strong [[mothball|mothball]] odor. Naphthalene is derived from coal tar. It consists of two aromatic rings attached on one side. Naphthalene was formerly used as a dusting powder, [[insecticide|insecticide]], [[fungicide|fungicide]], and moth repellent. It is not recommended for general use as an insecticide because it may recrystallize on specimens and can discolor wool in the presence of moisture. Additionally, its actual effectiveness against insects is questionable. It is used industrially as an intermediate in making [[dye|dyes]], explosives, and [[synthetic%20resin|synthetic resins]]. Naphthalene can sublime at room temperature. |
| + | [[[SliderGallery rightalign|naphthalene.jpg~Chemical structure]]] | ||
| + | == Synonyms and Related Terms == | ||
| − | + | naphthalin; naphthene; tar camphor; mothballs; moth crystals; white tar; Moth Flakes; Naphthalin (Deut.); naftalina (Esp.); naphtalène (Fr.); naftalene (It.); naftaleen (Ned.); naftalen (Pol., Sven.) | |
| − | + | ==Risks == | |
| − | [ | + | * Toxic by ingestion, inhalation and skin absorption. |
| + | * May cause allergic reactions. | ||
| + | * Natural resins are softened by naphthalene. | ||
| + | * Can corrode metals. | ||
| + | * Combustible. Flash point = 87C (189F) | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AA3334718&productDescription=NAPHTHALENE+99.6%25+50G&vendorId=VN00024248&countryCode=US&language=en SDS] | ||
| − | == | + | == Physical and Chemical Properties == |
| − | Fluoresces purple in mercury light. Soluble in chloroform, benzene, carbon disulfide, ether. Partially soluble in methanol and ethanol. Insoluble in water. | + | * Fluoresces purple in mercury light. |
| + | * Soluble in chloroform, benzene, carbon disulfide, ether. Partially soluble in methanol and ethanol. Insoluble in water. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 22: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 80.2 | + | | 80.2 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.162 | + | | 1.162 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 39: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 217.9 | + | | 217.9 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * M.Hayward, "Naphthalene" ''Conservation News'', No. 49, pp. 40-43, 1992. | |
| − | * | + | * Lynda A. Zycherman, J.Richard Schrock, ''A Guide to Museum Pest Control'', FAIC and Association of Systematics Collections, Washington DC, 1988 |
| − | * | + | * J. Dawson, ''CCI Technical Bulletin'', 'Solving Museum Insect Problems: Chemical Control' , Canadian Conservation Institute, Ottawa, No. 15 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6457 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6457 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Naphthalene Accessed Jan. 6, 2006 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * | + | * Marjorie Shelley, ''The Care and Handling of Art Objects'', The Metropolitan Museum, New York, 1987 |
| − | * | + | * Hoechst Celanese Corporation, ''Dictionary of Fiber & Textile Technology'' (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 09:58, 19 October 2022
Description
White, crystalline flakes with a strong Mothball odor. Naphthalene is derived from coal tar. It consists of two aromatic rings attached on one side. Naphthalene was formerly used as a dusting powder, Insecticide, Fungicide, and moth repellent. It is not recommended for general use as an insecticide because it may recrystallize on specimens and can discolor wool in the presence of moisture. Additionally, its actual effectiveness against insects is questionable. It is used industrially as an intermediate in making dyes, explosives, and synthetic resins. Naphthalene can sublime at room temperature.
Synonyms and Related Terms
naphthalin; naphthene; tar camphor; mothballs; moth crystals; white tar; Moth Flakes; Naphthalin (Deut.); naftalina (Esp.); naphtalène (Fr.); naftalene (It.); naftaleen (Ned.); naftalen (Pol., Sven.)
Risks
- Toxic by ingestion, inhalation and skin absorption.
- May cause allergic reactions.
- Natural resins are softened by naphthalene.
- Can corrode metals.
- Combustible. Flash point = 87C (189F)
- ThermoFisher: SDS
Physical and Chemical Properties
- Fluoresces purple in mercury light.
- Soluble in chloroform, benzene, carbon disulfide, ether. Partially soluble in methanol and ethanol. Insoluble in water.
| Composition | C10H8 |
|---|---|
| CAS | 91-20-3 |
| Melting Point | 80.2 C |
| Density | 1.162 g/ml |
| Molecular Weight | mol. wt. = 128.16 |
| Boiling Point | 217.9 C |
Resources and Citations
- M.Hayward, "Naphthalene" Conservation News, No. 49, pp. 40-43, 1992.
- Lynda A. Zycherman, J.Richard Schrock, A Guide to Museum Pest Control, FAIC and Association of Systematics Collections, Washington DC, 1988
- J. Dawson, CCI Technical Bulletin, 'Solving Museum Insect Problems: Chemical Control' , Canadian Conservation Institute, Ottawa, No. 15
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6457
- Wikipedia: http://en.wikipedia.org/wiki/Naphthalene Accessed Jan. 6, 2006
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Marjorie Shelley, The Care and Handling of Art Objects, The Metropolitan Museum, New York, 1987
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990