Difference between revisions of "Ferrous sulfate"
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[File:iron sulfate_1.jpg|thumb|Powdered iron sulfate]] | [[File:iron sulfate_1.jpg|thumb|Powdered iron sulfate]] | ||
== Description == | == Description == | ||
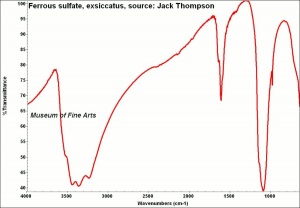
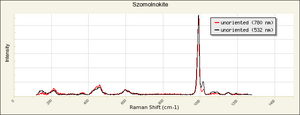
| − | + | [[[SliderGallery rightalign|FerroussulfateThompsonIR.jpg~FTIR|Szomolnokite Raman RRUFF R070620.png~Raman (RRUFF)]]] | |
A blue-green crystalline material that has been used in inks, dyes, and the manufacture of iron. Ferrous sulfate oxidizes slowly in moist air to form a brown coating of basic ferric sulfate. In dry conditions, the salt effloresces. Ferrous sulfate is used as a [[mordant]] in textile dyeing where is termed a saddening agent because it dulls or darkens the dye color. Excess exposure to wool leaves the fibers stiff and harsh. Ferrous sulfate has also been added to some inks as it made them blacker when it decomposed to [[ferrous oxide]]. However, this process gives off [[sulfuric acid]] that can degrade the substrate. The same reaction proved deleterious to many leathers that were sprinkled with ferrous sulfate to produce markings, such as sprinkled calf and mottled calf. Ferrous sulfate occurs naturally in the following crystals: szomolnokite [FeSO4.H2O], rozenite [FeSO4.4H2O], siderotil [FeSO4.5H2O], and [[melanterite]] [FeSO4.7H2O]. | A blue-green crystalline material that has been used in inks, dyes, and the manufacture of iron. Ferrous sulfate oxidizes slowly in moist air to form a brown coating of basic ferric sulfate. In dry conditions, the salt effloresces. Ferrous sulfate is used as a [[mordant]] in textile dyeing where is termed a saddening agent because it dulls or darkens the dye color. Excess exposure to wool leaves the fibers stiff and harsh. Ferrous sulfate has also been added to some inks as it made them blacker when it decomposed to [[ferrous oxide]]. However, this process gives off [[sulfuric acid]] that can degrade the substrate. The same reaction proved deleterious to many leathers that were sprinkled with ferrous sulfate to produce markings, such as sprinkled calf and mottled calf. Ferrous sulfate occurs naturally in the following crystals: szomolnokite [FeSO4.H2O], rozenite [FeSO4.4H2O], siderotil [FeSO4.5H2O], and [[melanterite]] [FeSO4.7H2O]. | ||
| Line 7: | Line 7: | ||
Heptahydrate: green vitriol; iron vitriol; green copperas; colcothar of vitriol; copperas; sal chalybis; Feosol; Ironate; Mol-Iron; iron sulfate; iron (II) sulfate; iron mordant; martial vitriol; English vitriol; vitriol of Mars; ferrous sulphate (Br.); | Heptahydrate: green vitriol; iron vitriol; green copperas; colcothar of vitriol; copperas; sal chalybis; Feosol; Ironate; Mol-Iron; iron sulfate; iron (II) sulfate; iron mordant; martial vitriol; English vitriol; vitriol of Mars; ferrous sulphate (Br.); | ||
| − | |||
| − | |||
== Risks == | == Risks == | ||
| − | Irritating to skins, eyes, nose and throat. Produces sulfur oxide fumes when fired. | + | * Irritating to skins, eyes, nose and throat. |
| − | + | * Produces sulfur oxide fumes when fired. | |
| − | Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-f/S25325A.pdf SDS] | + | * Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-f/S25325A.pdf SDS] |
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 28: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 64 | + | | 64 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.897 | + | | 1.897 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
Latest revision as of 10:36, 9 December 2022
Description
A blue-green crystalline material that has been used in inks, dyes, and the manufacture of iron. Ferrous sulfate oxidizes slowly in moist air to form a brown coating of basic ferric sulfate. In dry conditions, the salt effloresces. Ferrous sulfate is used as a Mordant in textile dyeing where is termed a saddening agent because it dulls or darkens the dye color. Excess exposure to wool leaves the fibers stiff and harsh. Ferrous sulfate has also been added to some inks as it made them blacker when it decomposed to Ferrous oxide. However, this process gives off Sulfuric acid that can degrade the substrate. The same reaction proved deleterious to many leathers that were sprinkled with ferrous sulfate to produce markings, such as sprinkled calf and mottled calf. Ferrous sulfate occurs naturally in the following crystals: szomolnokite [FeSO4.H2O], rozenite [FeSO4.4H2O], siderotil [FeSO4.5H2O], and Melanterite [FeSO4.7H2O].
Synonyms and Related Terms
Heptahydrate: green vitriol; iron vitriol; green copperas; colcothar of vitriol; copperas; sal chalybis; Feosol; Ironate; Mol-Iron; iron sulfate; iron (II) sulfate; iron mordant; martial vitriol; English vitriol; vitriol of Mars; ferrous sulphate (Br.);
Risks
- Irritating to skins, eyes, nose and throat.
- Produces sulfur oxide fumes when fired.
- Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in water. Insoluble in ethanol. Oxidized slowly by moisture; rate increased by heat, alkalis, and light. Incompatible with alkalis, Au and Ag salts, KI, Na tartrate, Na borate, tannins. Ferrous sulfate is used in a qualitative 'brown ring' test for nitrates.
| Composition | FeSO4 |
|---|---|
| CAS | 7720-78-7 |
| Melting Point | 64 C |
| Density | 1.897 g/ml |
| Molecular Weight | mol. wt. = 151.91 |
| Refractive Index | 1.802, 1.814, 1.818 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Palmy Weigle, Ancient Dyes for Modern Weavers, Watson-Guptill Publications, New York, 1974
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4105
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.802, 1.814, 1.818