Difference between revisions of "Aragonite"
(username removed) |
|||
| (12 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:1993.818-SC36608.jpg|thumb|]] | + | [[File:1993.818-SC36608.jpg|thumb|Lizard effigy vase<br>MFA# 1993.818]] |
== Description == | == Description == | ||
| − | A crystalline form of [ | + | A crystalline form of [[calcium%20carbonate|calcium carbonate]] that occurs in [[coral|coral]], [[seashell|shells]], [[pearl|pearls]], stalactites, and water deposits. Aragonite was named after the Aragon region in Spain where it was first discovered. Its orthorhombic system forms compact, acicular crystals that make it harder and heavier than [[calcite|calcite]]. When aragonite is formed by water deposition of calcium carbonate, the crystals often grow in radiating flowers. Aragonite mines are located in Europe, Bolivia, and the U.S. (New Mexico, Arizona). Aragonite was used in antiquity for beads and decorative items. It can be converted to calcite with heat (470 C) and changes slowly to calcite at room temperature. |
| − | [[File: | + | [[File:Shell trumpet.jpg|thumb|Shell trumpet<br>MFA# 17.2170]] |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | |||
calcium carbonate; nacre; shell white; coral; Aragonit (Deut., Pol.); aragonita (Esp.); aragonito (Esp.); aragonite (Fr., Port.); aragoniet (Ned.) | calcium carbonate; nacre; shell white; coral; Aragonit (Deut., Pol.); aragonita (Esp.); aragonito (Esp.); aragonite (Fr., Port.); aragoniet (Ned.) | ||
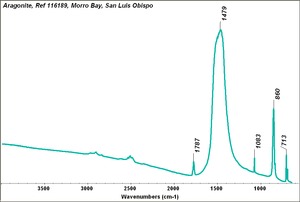
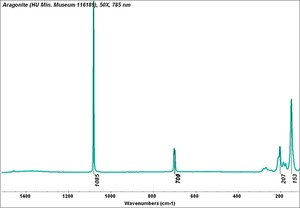
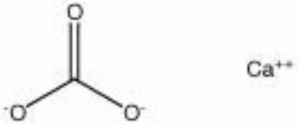
| + | [[[SliderGallery rightalign|Aragonite 2.TIF~FTIR (MFA)|Aragonite (HU Min. Museum 116189), 50X, 785 nm copy.tif~Raman (MFA)|aragonite.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| − | [ | + | * No significant hazards. |
| − | + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC423510025&productDescription=CALCIUM+CARBONATE+99%2BACS+2.5KG&vendorId=VN00032119&countryCode=US&language=en SDS] | |
| − | == | + | == Physical and Chemical Properties == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Orthorhombic crystal system with platy or fibrous, acicular crystals that are often twinned | |
| + | * Reacts with acids to evolve carbon dioxide. | ||
| + | * Aragonite is harder and denser than calcite. | ||
| + | * Fracture = subconchoidal, brittle | ||
| + | * Luster = vitreous to resinous | ||
| + | * Transparent to translucent | ||
| + | * Streak = white | ||
| + | * Fluorescence = variable | ||
| + | * Birefringence = strong showing interference colors | ||
| + | * Straight extinction | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 23: | Line 29: | ||
! scope="row"| Composition | ! scope="row"| Composition | ||
| CaCO3 | | CaCO3 | ||
| − | |||
| − | |||
| − | |||
|- | |- | ||
! scope="row"| Mohs Hardness | ! scope="row"| Mohs Hardness | ||
| Line 31: | Line 34: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.93-2.95 | + | | 2.93-2.95 g/ml |
| − | |||
| − | |||
| − | |||
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.530, 1.682, 1.686 | | 1.530, 1.682, 1.686 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 55: | Line 45: | ||
File:Aragoniteemr.jpg|Aragonite | File:Aragoniteemr.jpg|Aragonite | ||
File:pa20720aragonite.jpg|Aragonite | File:pa20720aragonite.jpg|Aragonite | ||
| + | File:36.178-20-3.jpg|Stamp seal<br>MFA# 36.178 | ||
</gallery> | </gallery> | ||
| + | == Resources and Citations == | ||
| + | * R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", ''Artists Pigments'', Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993. | ||
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Aragonite.shtml Aragonite] | |
| − | + | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | |
| − | * | ||
| − | * | + | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.529-1.530; beta=1.680-1.682; gamma=1.685-1.686 |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: aragonite" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: aragonite" [Accessed December 4, 2001 |
| − | * | + | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Aragonite (Accessed Aug. 30 2005) |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 131 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 10:28, 17 December 2022
Description
A crystalline form of Calcium carbonate that occurs in Coral, shells, pearls, stalactites, and water deposits. Aragonite was named after the Aragon region in Spain where it was first discovered. Its orthorhombic system forms compact, acicular crystals that make it harder and heavier than Calcite. When aragonite is formed by water deposition of calcium carbonate, the crystals often grow in radiating flowers. Aragonite mines are located in Europe, Bolivia, and the U.S. (New Mexico, Arizona). Aragonite was used in antiquity for beads and decorative items. It can be converted to calcite with heat (470 C) and changes slowly to calcite at room temperature.
Synonyms and Related Terms
calcium carbonate; nacre; shell white; coral; Aragonit (Deut., Pol.); aragonita (Esp.); aragonito (Esp.); aragonite (Fr., Port.); aragoniet (Ned.)
Risks
- No significant hazards.
- ThermoFisher: SDS
Physical and Chemical Properties
- Orthorhombic crystal system with platy or fibrous, acicular crystals that are often twinned
- Reacts with acids to evolve carbon dioxide.
- Aragonite is harder and denser than calcite.
- Fracture = subconchoidal, brittle
- Luster = vitreous to resinous
- Transparent to translucent
- Streak = white
- Fluorescence = variable
- Birefringence = strong showing interference colors
- Straight extinction
| Composition | CaCO3 |
|---|---|
| Mohs Hardness | 3.5 - 4.0 |
| Density | 2.93-2.95 g/ml |
| Refractive Index | 1.530, 1.682, 1.686 |
Additional Images
Resources and Citations
- R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", Artists Pigments, Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993.
- Mineralogy Database: Aragonite
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.529-1.530; beta=1.680-1.682; gamma=1.685-1.686
- Encyclopedia Britannica, http://www.britannica.com Comment: aragonite" [Accessed December 4, 2001
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: http://en.wikipedia.org/wiki/Aragonite (Accessed Aug. 30 2005)
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 131
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998