Difference between revisions of "Emerald"
| (One intermediate revision by the same user not shown) | |||
| Line 6: | Line 6: | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
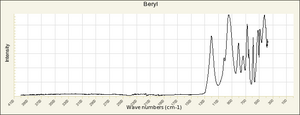
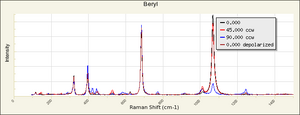
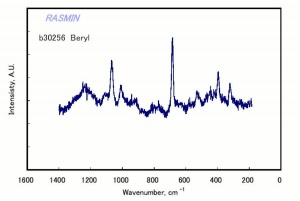
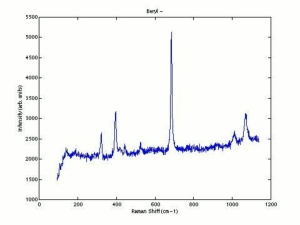
| − | + | [[[SliderGallery rightalign|Beryl IR-ATR RRUFF 40002.png~IR-ATR (RRUFF)|Beryl raman RRUFF 40002.png~Raman (RRUFF)|berylRS.jpg~Raman (RASMIN)|Berylitaly1.jpg~Raman (U of Parma)]]] | |
green beryl; beryllium aluminum silicate; smaragd (Dan., Ned., Nor., Sven.); Smaragd (Deut.); esmeralda (Esp.); émeraude (Fr.); szmaragd (Pol.); esmeralda (Port.) | green beryl; beryllium aluminum silicate; smaragd (Dan., Ned., Nor., Sven.); Smaragd (Deut.); esmeralda (Esp.); émeraude (Fr.); szmaragd (Pol.); esmeralda (Port.) | ||
| + | == Risks == | ||
| + | * Beryllium is a known carcinogen. | ||
==Physical and Chemical Properties== | ==Physical and Chemical Properties== | ||
| − | * Hexagonal system with prismatic crystals | + | * Hexagonal system with prismatic crystals |
| − | * Cleavage is poor in one direction | + | * Cleavage is poor in one direction |
| − | + | * Fracture = uneven to conchoidal | |
| − | * Fracture = uneven to conchoidal | + | * Luster = vitreous to resinous |
| − | * Luster = vitreous | + | * Streak = colorless to white |
| − | * Streak = colorless | + | * Natural stones may have inclusions |
| − | * Natural stones may have inclusions | + | * Fluorescence = generally inert |
| + | * Pleochroism = moderate to strong; green and bluish green | ||
* Synthetic emeralds appear opaque and dull red in UV light | * Synthetic emeralds appear opaque and dull red in UV light | ||
| Line 26: | Line 29: | ||
|- | |- | ||
! scope="row"| Mohs Hardness | ! scope="row"| Mohs Hardness | ||
| − | | 7.5 - 8 | + | | 7.5 - 8.0 |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| Line 32: | Line 35: | ||
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| − | | 1. | + | | 1.577-1.583 |
| + | |- | ||
| + | ! scope="row"| Birefringence | ||
| + | | 0.005 - 0.009 | ||
|} | |} | ||
| Line 40: | Line 46: | ||
==Resources and Citations== | ==Resources and Citations== | ||
| − | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | |
* Sue Fuller, ''Rocks and Minerals'', DK Publishing, Inc., New York City, 1995 | * Sue Fuller, ''Rocks and Minerals'', DK Publishing, Inc., New York City, 1995 | ||
| − | |||
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
| − | |||
* A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | ||
| − | |||
* Yasukazu Suwa, ''Gemstones: Quality and Value, Volume 1'', Sekai Bunka Publishing Inc., Tokyo, 1999 Comment: RI=1.577-1.583; Specific gravity=2.72 | * Yasukazu Suwa, ''Gemstones: Quality and Value, Volume 1'', Sekai Bunka Publishing Inc., Tokyo, 1999 Comment: RI=1.577-1.583; Specific gravity=2.72 | ||
| − | + | * Michael O'Donoghue and Louise Joyner, ''Identification of Gemstones'', Butterworth-Heinemann, Oxford, 2003 Comment: Chivor emeralds: RI=1.571-1.574; 1.577-1.580; spec.grav.=2.67-2.71 Muzo emeralds:RI=1.576-1.580; 1.582-1.586; spec.grav.=2.71-2.72 | |
| − | * Michael O'Donoghue and Louise Joyner, ''Identification of Gemstones'', Butterworth-Heinemann, Oxford, 2003 Comment: Chivor emeralds:RI=1.571-1.574; 1.577-1.580; spec.grav.=2.67-2.71 Muzo emeralds:RI=1.576-1.580; 1.582-1.586; spec.grav.=2.71-2.72 | + | * Wikipedia: [https://en.wikipedia.org/wiki/Emerald Emerald] (Accessed Oct. 18, 2005 and Dec 2022) |
| − | |||
| − | * Wikipedia | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
Latest revision as of 14:05, 21 December 2022
Description
A transparent, green Gemstone composed of beryllium aluminum silicate. The prismatic Beryl crystals get their green color from trace amounts of Chromium or Vanadium. Emeralds were produced from Cleopatra's mines in Egypt's eastern desert in ancient times, but these stones were often flawed with microscopic elongated bubbles. Later, Romans made glass imitations of the stones because emeralds were ranked third in value after diamonds and pearls. High quality emeralds were imported from Columbia by the Spanish from the 1500s. The Muzo region, near Bogota, is still the main source for high quality emeralds. Other current sources for emeralds are the Urals, Austria (Habachtal), Norway, Australia, Tanzania, Zambia (discovered in 1931, produced since 1967), Zimbabwe (Sandawana, since 1956), Pakistan, India, Afghanistan, and Brazil. Emeralds tend to be brittle and crack easily. Since 1934, synthetic emeralds have been made from aquamarine seed crystals with a high-pressure, high-temperature process. They are used in lasers, masers, and semiconductors.
Synonyms and Related Terms
green beryl; beryllium aluminum silicate; smaragd (Dan., Ned., Nor., Sven.); Smaragd (Deut.); esmeralda (Esp.); émeraude (Fr.); szmaragd (Pol.); esmeralda (Port.)
Risks
- Beryllium is a known carcinogen.
Physical and Chemical Properties
- Hexagonal system with prismatic crystals
- Cleavage is poor in one direction
- Fracture = uneven to conchoidal
- Luster = vitreous to resinous
- Streak = colorless to white
- Natural stones may have inclusions
- Fluorescence = generally inert
- Pleochroism = moderate to strong; green and bluish green
- Synthetic emeralds appear opaque and dull red in UV light
| Composition | Be3Al2SiO6 |
|---|---|
| Mohs Hardness | 7.5 - 8.0 |
| Density | 2.68-2.78 g/ml |
| Refractive Index | 1.577-1.583 |
| Birefringence | 0.005 - 0.009 |
Comparisons
Properties of Common Gemstones
Resources and Citations
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- Sue Fuller, Rocks and Minerals, DK Publishing, Inc., New York City, 1995
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- Yasukazu Suwa, Gemstones: Quality and Value, Volume 1, Sekai Bunka Publishing Inc., Tokyo, 1999 Comment: RI=1.577-1.583; Specific gravity=2.72
- Michael O'Donoghue and Louise Joyner, Identification of Gemstones, Butterworth-Heinemann, Oxford, 2003 Comment: Chivor emeralds: RI=1.571-1.574; 1.577-1.580; spec.grav.=2.67-2.71 Muzo emeralds:RI=1.576-1.580; 1.582-1.586; spec.grav.=2.71-2.72
- Wikipedia: Emerald (Accessed Oct. 18, 2005 and Dec 2022)
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997