Difference between revisions of "Amber"
(username removed) |
|||
| (11 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:38.1396-SC153258.jpg|thumb| | + | [[File:38.1396-SC153258.jpg|thumb|Baltic amber statuetter<br>MFA# 38.1396]] |
== Description == | == Description == | ||
| − | + | [[File:02.217-E11498CR-d1.jpg|thumb|Amber earrings<br>MFA# 02.217]] | |
1) An obsolete name for any hard resin. | 1) An obsolete name for any hard resin. | ||
| − | 2) True amber is a yellowish, hard, glassy, fossil resin which is most commonly found in the region of the Baltic Sea. Types of amber classified by location include Baltic amber (succinite), Rumanian (rumanite), Sicilian (simetite) and Burmese amber (burmite). Amber is composed of complex mixtures of oxidized and polymerized resin acids and resin alcohols. Amber is broadly classified by composition as succinite amber when it contains [ | + | 2) True amber is a yellowish, hard, glassy, fossil resin which is most commonly found in the region of the Baltic Sea. Types of amber classified by location include Baltic amber (succinite), Rumanian (rumanite), Sicilian (simetite) and Burmese amber (burmite). Amber is composed of complex mixtures of oxidized and polymerized resin acids and resin alcohols. Amber is broadly classified by composition as succinite amber when it contains [[succinic%20acid|succinic acid]] (usually 3-8%: also called true amber) and retinite amber when it does not (Serpico and White 2000). Amber has been gathered or mined since Paleolithic times. Amber develops an electrical charge when rubbed with a cloth. It is easy to carve and was often used in its natural state for jewelry, beads, amulets, and small vessels. Amber was also used as an ingredient in paint. Dark color oil/amber varnishes are made by dissolving melted amber in amber oil, turpentine oil, or a drying oil. |
| − | + | [[File:Amberpendantskes.jpg|thumb|Amber pendant]] | |
| − | [[File: | + | [[File:Amberinsectkes.jpg|thumb|Amber with an insect]] |
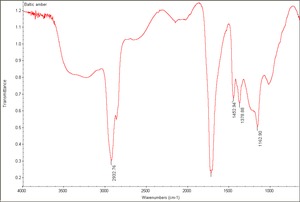
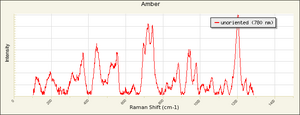
| + | [[[SliderGallery rightalign|Baltic amber.TIF~FTIR (MFA)|Amber Raman RRUFF R060569.png~Raman (RRUFF)]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
ambre (Fr.); ámbar (Esp.); rav (Dan., Nor.); simetita (Esp.); ambra (It.); barnsteen (Ned.); âmbar (Port.); bärnsten (Sven.); elektron (Gr.); Bernstein (Deut.); lyncurium; electrum; succinum; vernix; fornix; glassa; Bernice's Stone; berenice; resinite; retinite | ambre (Fr.); ámbar (Esp.); rav (Dan., Nor.); simetita (Esp.); ambra (It.); barnsteen (Ned.); âmbar (Port.); bärnsten (Sven.); elektron (Gr.); Bernstein (Deut.); lyncurium; electrum; succinum; vernix; fornix; glassa; Bernice's Stone; berenice; resinite; retinite | ||
| − | |||
| − | |||
Types include: succinite (Baltic, largest deposit); rumanite (Rumania); simetite (Sicily, retinite); burmite (Burma, usually red); allingite (Switzerland); beckerite (Baltic, dense and polishes poorly); delatynite (Russia); gedanite (Baltic, poor working properties); glessite (Baltic); krantzite (Saxony, soft); schraufite (Carpathian mt.); stantienite (Baltic, easily fractures); ambrite (New Zealand); amekit (Nigeria); chemawinite (Canada); Dominican republic amber (second largest deposit); hachettite (Italy); loban (near Mecca); Mexican retinite; North American amber | Types include: succinite (Baltic, largest deposit); rumanite (Rumania); simetite (Sicily, retinite); burmite (Burma, usually red); allingite (Switzerland); beckerite (Baltic, dense and polishes poorly); delatynite (Russia); gedanite (Baltic, poor working properties); glessite (Baltic); krantzite (Saxony, soft); schraufite (Carpathian mt.); stantienite (Baltic, easily fractures); ambrite (New Zealand); amekit (Nigeria); chemawinite (Canada); Dominican republic amber (second largest deposit); hachettite (Italy); loban (near Mecca); Mexican retinite; North American amber | ||
| − | + | == Risks == | |
| − | + | No significant hazards. | |
| − | + | == Physical and Chemical Properties == | |
| − | Saponification number = 115 | + | * Amber is very resistant to acids and alkalis and is not entirely soluble in any solvent |
| − | + | * A drop of ether will not affect the surface of true amber, but will leave other resins slightly sticky | |
| − | + | * Amber will float in a saturated salt solution | |
| + | * Succinites emit succinic acid when heated | ||
| + | * Saponification number = 115 | ||
| + | * Acid number = 15-35 | ||
| + | * Fracture = conchoidal | ||
| + | * Luster = waxy to resinous | ||
| + | * Fluorescence = variable; usually a strong yellowish green to bluish white in LW and weaker in SW | ||
| + | * Birefringence = none | ||
| + | * Amber is piezoelectric | ||
| + | * Inclusions may include gas bubble, flow lines, insects and other types of organic and inorganic materials | ||
| + | * Amber heat-treated in oil shows disk-like fractures called sun spangles | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 31: | Line 40: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | | + | | 200-300 C (forms oil of amber) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.05-1. | + | | 1.05 - 1.10 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.54 - 1.55 | | 1.54 - 1.55 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 52: | Line 53: | ||
[[media:download_file_100.pdf|Properties of Natural Resins]] | [[media:download_file_100.pdf|Properties of Natural Resins]] | ||
| − | + | == Resources and Citations == | |
| − | |||
| − | |||
| − | |||
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | * David A Grimaldi, ''Amber:Window to the Past'', The American Museum of Natural History with Harry N. Abrams, New York, 1996. | ||
| + | * D.Thickett, P.Cruickshank, C.Ward, "The Conservation of Amber" ''Studies in Conservation'', 40:217-226, 1995. | ||
| + | * M.Serpico, R.White, "Resins, Amber and Bitumen" in ''Ancient Egyptian Materials and Technology'', P.Nicholson, I.Shaw (eds.), Cambridge University Press, 2000, p. 430-474. | ||
| + | * Ancient Trade Routes: [http://www.ancientroute.com/resource/stone/amber.htm Website] | ||
| + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: ref. index = 1.55 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: ref. index = 1.55 | ||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: Amber | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: Amber | ||
| − | |||
* Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | ||
| − | |||
* Tom Rowland, Noel Riley, ''A-Z Guide to Cleaning, Conserving and Repairing Antiques'', Constable and Co., Ltd., London, 1981 | * Tom Rowland, Noel Riley, ''A-Z Guide to Cleaning, Conserving and Repairing Antiques'', Constable and Co., Ltd., London, 1981 | ||
| − | |||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | + | * Oppi Untracht, ''Jewelry Concepts and Technology'', Doubleday & Co., Inc., New York City, 1985 Comment:specific gravity = 1.08-1.10; Mohs hardness= 2.5-3.0; Refractive index= 1.54 | |
| − | * Oppi Untracht, ''Jewelry Concepts and Technology'', Doubleday & Co., Inc., New York City, 1985 | ||
| − | |||
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
| − | |||
* A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | ||
| − | |||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.06-1.11; ref. index=1.546 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.06-1.11; ref. index=1.546 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Amber Amber] Accessed Dec 2022 | |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 10:37, 3 January 2023
Description
1) An obsolete name for any hard resin.
2) True amber is a yellowish, hard, glassy, fossil resin which is most commonly found in the region of the Baltic Sea. Types of amber classified by location include Baltic amber (succinite), Rumanian (rumanite), Sicilian (simetite) and Burmese amber (burmite). Amber is composed of complex mixtures of oxidized and polymerized resin acids and resin alcohols. Amber is broadly classified by composition as succinite amber when it contains Succinic acid (usually 3-8%: also called true amber) and retinite amber when it does not (Serpico and White 2000). Amber has been gathered or mined since Paleolithic times. Amber develops an electrical charge when rubbed with a cloth. It is easy to carve and was often used in its natural state for jewelry, beads, amulets, and small vessels. Amber was also used as an ingredient in paint. Dark color oil/amber varnishes are made by dissolving melted amber in amber oil, turpentine oil, or a drying oil.
Synonyms and Related Terms
ambre (Fr.); ámbar (Esp.); rav (Dan., Nor.); simetita (Esp.); ambra (It.); barnsteen (Ned.); âmbar (Port.); bärnsten (Sven.); elektron (Gr.); Bernstein (Deut.); lyncurium; electrum; succinum; vernix; fornix; glassa; Bernice's Stone; berenice; resinite; retinite
Types include: succinite (Baltic, largest deposit); rumanite (Rumania); simetite (Sicily, retinite); burmite (Burma, usually red); allingite (Switzerland); beckerite (Baltic, dense and polishes poorly); delatynite (Russia); gedanite (Baltic, poor working properties); glessite (Baltic); krantzite (Saxony, soft); schraufite (Carpathian mt.); stantienite (Baltic, easily fractures); ambrite (New Zealand); amekit (Nigeria); chemawinite (Canada); Dominican republic amber (second largest deposit); hachettite (Italy); loban (near Mecca); Mexican retinite; North American amber
Risks
No significant hazards.
Physical and Chemical Properties
- Amber is very resistant to acids and alkalis and is not entirely soluble in any solvent
- A drop of ether will not affect the surface of true amber, but will leave other resins slightly sticky
- Amber will float in a saturated salt solution
- Succinites emit succinic acid when heated
- Saponification number = 115
- Acid number = 15-35
- Fracture = conchoidal
- Luster = waxy to resinous
- Fluorescence = variable; usually a strong yellowish green to bluish white in LW and weaker in SW
- Birefringence = none
- Amber is piezoelectric
- Inclusions may include gas bubble, flow lines, insects and other types of organic and inorganic materials
- Amber heat-treated in oil shows disk-like fractures called sun spangles
| Mohs Hardness | 2.5 - 3.0 |
|---|---|
| Melting Point | 200-300 C (forms oil of amber) |
| Density | 1.05 - 1.10 g/ml |
| Refractive Index | 1.54 - 1.55 |
Comparisons
Resources and Citations
- David A Grimaldi, Amber:Window to the Past, The American Museum of Natural History with Harry N. Abrams, New York, 1996.
- D.Thickett, P.Cruickshank, C.Ward, "The Conservation of Amber" Studies in Conservation, 40:217-226, 1995.
- M.Serpico, R.White, "Resins, Amber and Bitumen" in Ancient Egyptian Materials and Technology, P.Nicholson, I.Shaw (eds.), Cambridge University Press, 2000, p. 430-474.
- Ancient Trade Routes: Website
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: ref. index = 1.55
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: Amber
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Oppi Untracht, Jewelry Concepts and Technology, Doubleday & Co., Inc., New York City, 1985 Comment:specific gravity = 1.08-1.10; Mohs hardness= 2.5-3.0; Refractive index= 1.54
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.06-1.11; ref. index=1.546
- Wikipedia: Amber Accessed Dec 2022