Difference between revisions of "Polyvinylidene chloride"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:Baby hairs saran.jpg|thumb|Baby Hairs <br>MFA# 2012.314]] | ||
== Description == | == Description == | ||
| − | A thermoplastic polymer | + | A thermoplastic polymer with a common name of [[Saran film|Saran]], polyvinylidene chloride (PVDC) is made by polymerizing vinylidene chloride with monomers such as acrylic esters and unsaturated carboxyl groups to form long chains of vinylidene chloride. PVDC forms films that are impermeable to vapors, moisture, and oxygen. It is most often used as a barrier film for packaging. It was originally developed by the Dow Chemical Company, but the brand was sold to S. C. Johnson in 1988. In an eco-minded manufacturing step, in 2004, SC Johnson modified the formulation of 'Saran Wrap' from PVDC to [[polyethylene]]. PE has a higher permeability to oxygen than PVDC. |
| − | + | It is important to note that the term ‘[[bubble wrap]]’ is applied generically to many brands of film containing sealed air pockets. While the brand name Bubble Wrap is made from polyethylene, some brands are still manufactured using PVDC and PVC. All ‘bubble wrap’ films cannot be assumed to be polyethylene, and PVDC bubble wraps should be avoided. | |
| + | |||
| + | PVDC is on the ILFI [[Red list of Materials|Red list]] of building materials. | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
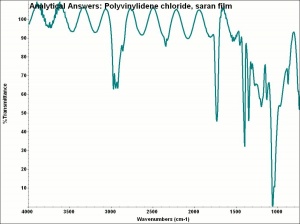
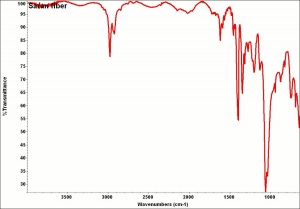
| − | + | [[[SliderGallery rightalign|aaiSARAN.jpg~FTIR|Saranfiberkj1.jpg~FTIR]]] | |
poly(vinylidene chloride); PVDC; poli(cloruro de vinilideno) (Esp.); chlorure de polyvinylidène (Fr.); polivinilidene cloruro (It.); cloreto de polivinilideno (Port.) | poly(vinylidene chloride); PVDC; poli(cloruro de vinilideno) (Esp.); chlorure de polyvinylidène (Fr.); polivinilidene cloruro (It.); cloreto de polivinilideno (Port.) | ||
| − | Examples: saran; Cryovac | + | Examples: saran (Dow Chemical [historic and made from PVDC], SC Johnson [current but their saran is made from polyethylene]); Cryovac (Sealed Air); Diofan (Solvay); Ixan (Solvay); Kreholan (Kureha), some [[Bubble wrap|bubble wraps]]. |
| − | + | == Applications == | |
| + | * Barrier coating to protect movement of moisture and oxygen. | ||
| − | == | + | == Personal Risks == |
| + | May release hydrochloric acid when heated. This can damage the lungs. | ||
| − | + | == Collection Risks == | |
| + | May release hydrochloric acid when heated and in the presence of moisture (Hatchfield, 2002). Can damage metals and organics. Plasticizers often used in PVDC films, such as dialkyl phthalates, can migrate to the surface. These plasticizers can solubilize resins, including some artifact coatings (e.g. cellulose nitrate) (Ibid). The presence of plasticizers in PVC based films lowers the resistance of the material to attack by oxidizing acids, potentially accelerating aforementioned degradation processes (Lampman, 2003). | ||
| − | + | == Environment Risks == | |
| + | Combustible but self-extinguishing. Vinylidene chloride polymers undergo thermal decomposition in two distinct stages. The first occurs at very modest temperature (120-160 °C) and corresponds to the elimination of hydrogen chloride. This degradative dehydrohalogenation is initiated at dichloromethylene units present in the polymers as a consequence of interaction with a variety of agents. This initial degradation corresponds to the loss of hydrogen chloride uncomplicated by other processes. | ||
| − | + | == Physical and Chemical Properties == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Burns with green flame producing pungent odor and evolving HCl. | |
| − | + | * Turns yellow or brown when exposed to alkalis. | |
| − | + | * Melting Point = 185-200 | |
| + | * Density = 1.86-1.88 | ||
== Comparisons == | == Comparisons == | ||
| Line 38: | Line 39: | ||
[[media:download_file_381.pdf|General Characteristics of Polymers]] | [[media:download_file_381.pdf|General Characteristics of Polymers]] | ||
| − | + | == Resources And Citations == | |
| − | + | * https://www.thoughtco.com/history-of-pvdc-4070927 | |
| − | == | + | * Howell, Bobby. (2015). Mechanism of Thermal Degradation of Vinylidene Chloride Barrier Polymers. doi:10.1002/9781119117711. ch9. Pp. 209-220. |
| + | * Hatchfield, Pamela. Pollutants In The Museum Environment. London: Archetype Publications, 2002. | ||
| + | * Lampman, Steve. Characterization and Failure Analysis of Plastics. ASM International, 2003 | ||
| + | * Contributions: Catherine Stephens, AIC Plastics Panel, 2020 | ||
| + | * Contributions: Paige Schmidt, AIC Plastics Panel, 2020 | ||
* Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | ||
Latest revision as of 13:09, 3 November 2023
Description
A thermoplastic polymer with a common name of Saran, polyvinylidene chloride (PVDC) is made by polymerizing vinylidene chloride with monomers such as acrylic esters and unsaturated carboxyl groups to form long chains of vinylidene chloride. PVDC forms films that are impermeable to vapors, moisture, and oxygen. It is most often used as a barrier film for packaging. It was originally developed by the Dow Chemical Company, but the brand was sold to S. C. Johnson in 1988. In an eco-minded manufacturing step, in 2004, SC Johnson modified the formulation of 'Saran Wrap' from PVDC to Polyethylene. PE has a higher permeability to oxygen than PVDC.
It is important to note that the term ‘Bubble wrap’ is applied generically to many brands of film containing sealed air pockets. While the brand name Bubble Wrap is made from polyethylene, some brands are still manufactured using PVDC and PVC. All ‘bubble wrap’ films cannot be assumed to be polyethylene, and PVDC bubble wraps should be avoided.
PVDC is on the ILFI Red list of building materials.
Synonyms and Related Terms
poly(vinylidene chloride); PVDC; poli(cloruro de vinilideno) (Esp.); chlorure de polyvinylidène (Fr.); polivinilidene cloruro (It.); cloreto de polivinilideno (Port.)
Examples: saran (Dow Chemical [historic and made from PVDC], SC Johnson [current but their saran is made from polyethylene]); Cryovac (Sealed Air); Diofan (Solvay); Ixan (Solvay); Kreholan (Kureha), some bubble wraps.
Applications
- Barrier coating to protect movement of moisture and oxygen.
Personal Risks
May release hydrochloric acid when heated. This can damage the lungs.
Collection Risks
May release hydrochloric acid when heated and in the presence of moisture (Hatchfield, 2002). Can damage metals and organics. Plasticizers often used in PVDC films, such as dialkyl phthalates, can migrate to the surface. These plasticizers can solubilize resins, including some artifact coatings (e.g. cellulose nitrate) (Ibid). The presence of plasticizers in PVC based films lowers the resistance of the material to attack by oxidizing acids, potentially accelerating aforementioned degradation processes (Lampman, 2003).
Environment Risks
Combustible but self-extinguishing. Vinylidene chloride polymers undergo thermal decomposition in two distinct stages. The first occurs at very modest temperature (120-160 °C) and corresponds to the elimination of hydrogen chloride. This degradative dehydrohalogenation is initiated at dichloromethylene units present in the polymers as a consequence of interaction with a variety of agents. This initial degradation corresponds to the loss of hydrogen chloride uncomplicated by other processes.
Physical and Chemical Properties
- Burns with green flame producing pungent odor and evolving HCl.
- Turns yellow or brown when exposed to alkalis.
- Melting Point = 185-200
- Density = 1.86-1.88
Comparisons
Physical Properties for Selected Thermoplastic Resins
General Characteristics of Polymers
Resources And Citations
- https://www.thoughtco.com/history-of-pvdc-4070927
- Howell, Bobby. (2015). Mechanism of Thermal Degradation of Vinylidene Chloride Barrier Polymers. doi:10.1002/9781119117711. ch9. Pp. 209-220.
- Hatchfield, Pamela. Pollutants In The Museum Environment. London: Archetype Publications, 2002.
- Lampman, Steve. Characterization and Failure Analysis of Plastics. ASM International, 2003
- Contributions: Catherine Stephens, AIC Plastics Panel, 2020
- Contributions: Paige Schmidt, AIC Plastics Panel, 2020
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000