Difference between revisions of "Cyanoacrylate resin"
(username removed) |
|||
| (25 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A thermoplastic polymer commonly used as a fast-setting, strong adhesive. The first cyanoacrylate adhesive was made in 1941, but not marketed till 1958 as Eastman | + | A thermoplastic polymer commonly used as a fast-setting, strong adhesive. The first cyanoacrylate adhesive was made in 1941, but not marketed till 1958 as Eastman 910® [Eastman Kodak]. Cyanoacrylate adhesives are based on ethyl-2-cyanoacrylate polymers. Most commercial formulations also contain stabilizers, thickeners and catalysts. The glues set rapidly (5 seconds - 3 minutes) upon exposure to ultraviolet radiation or moisture. When cured, they form an extremely strong bond that is fairly insoluble. Cyanoacrylate glues has been used for gluing glass, ceramics and other hard materials. They also have medical and dental applications to suture skin and weld crowns. Some cyanoacrylate glues may lose adhesive strength with time. Ultraviolet light and contact with alkaline materials (glass and some stones) will accelerate the degradation process. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | CA; super glue; instant adhesive; Super Glue Gel [Loctite]; | + | CA; super glue; instant adhesive; Super Glue Gel [Loctite]; Krazy® glue [Borden]; Super Attack [Loctite]; Zap; Eastman 910® [Eastman Chemical]; ELFY® super glue; ethyl cyanoacrylate; Dermabond; Traumaseal; cianoacrilati (It.); cyanoacrylaat (Ned.); resina de cianoacrilato (Esp.) |
| − | [ | + | == Applications == |
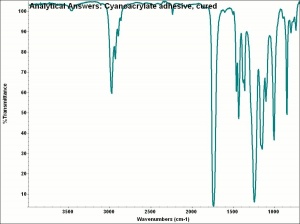
| − | + | [[[SliderGallery rightalign|aaiCYANOacrylate.jpg~FTIR]]] | |
| − | + | * Adhesive | |
| − | + | == Personal Risks == | |
| − | + | May cause irritation to skin and eyes. Will adhere objects on contact, including skin. Flash points range from 75-80°C. | |
| − | + | == Environmental Risks == | |
| + | Formaldehyde degradation product (more so in ethyl type).<ref name=Jane>Jane L. Down and Elzbieta Kaminska, "A Preliminary Study of the Degradation of Cyanoacrylate Adhesives in the Presence and Absence of Fossil Material", ''Journal of Vertebrate Paleontology'', Vol. 26, No. 3 (Sep. 11, 2006), pp. 519-525</ref> | ||
| − | + | == Collection Risks == | |
| − | + | * Hydrolysis degradation can occur in alkaline conditions (glass surfaces).<ref name=Jane/>, <ref name=IIC>A literature review of cyanoacrylate adhesives. Reviews in conservation no. 2 (2001), pp. 35-38 International Institute for Conservation of Historic and Artistic Works, London, United Kingdom</ref> | |
| − | + | * Cyanoacrylates undergo ultraviolet degradation.<ref name=Jane/>, <ref name=IIC/> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | == Physical and Chemical Properties == |
| − | + | * Uncured glue soluble in acetone, methanol, toluene, MEK. | |
| + | * Cured glue is slightly soluble in DMF or nitromethane. | ||
| + | * Soaking in acetone may decrease adhesion and cause the join to separate. | ||
| + | * CAS = 7085-85-0 | ||
| + | * Density = 1.05-1.10 | ||
| − | == | + | == Working Properties == |
| + | Cures quickly | ||
| − | + | == Forms/Sizes == | |
| + | Often sold in small container with application device | ||
== Comparisons == | == Comparisons == | ||
| Line 40: | Line 42: | ||
[[media:download_file_336.pdf|General Characteristics of Polymers]] | [[media:download_file_336.pdf|General Characteristics of Polymers]] | ||
| + | == Resources and Citations == | ||
| + | <references /> | ||
| + | |||
| + | * Contributions: Kate Wight Tyler, AIC Plastics Panel, 2020. | ||
| + | * H.W.Coover, J.M.McIntire, "Cyanoacrylate Adhesives" in ''Handbook of Adhesives'', I.Skeist (ed.), Van Nostrand Reinhold, New York, 1977, p.569-580. | ||
| − | + | * Website: [http://www.speedline.ca/edavidson/cyanoacrylates.html www.speedline.ca/edavidson/cyanoacrylates.html] | |
| − | * | + | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Irving Skeist, ''Handbook of Adhesives'', Van Nostrand Reinhold Company, New York, 1977 |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Cyanoacrylate (Accessed Feb. 10, 2006) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 07:20, 7 July 2020
Description
A thermoplastic polymer commonly used as a fast-setting, strong adhesive. The first cyanoacrylate adhesive was made in 1941, but not marketed till 1958 as Eastman 910® [Eastman Kodak]. Cyanoacrylate adhesives are based on ethyl-2-cyanoacrylate polymers. Most commercial formulations also contain stabilizers, thickeners and catalysts. The glues set rapidly (5 seconds - 3 minutes) upon exposure to ultraviolet radiation or moisture. When cured, they form an extremely strong bond that is fairly insoluble. Cyanoacrylate glues has been used for gluing glass, ceramics and other hard materials. They also have medical and dental applications to suture skin and weld crowns. Some cyanoacrylate glues may lose adhesive strength with time. Ultraviolet light and contact with alkaline materials (glass and some stones) will accelerate the degradation process.
Synonyms and Related Terms
CA; super glue; instant adhesive; Super Glue Gel [Loctite]; Krazy® glue [Borden]; Super Attack [Loctite]; Zap; Eastman 910® [Eastman Chemical]; ELFY® super glue; ethyl cyanoacrylate; Dermabond; Traumaseal; cianoacrilati (It.); cyanoacrylaat (Ned.); resina de cianoacrilato (Esp.)
Applications
- Adhesive
Personal Risks
May cause irritation to skin and eyes. Will adhere objects on contact, including skin. Flash points range from 75-80°C.
Environmental Risks
Formaldehyde degradation product (more so in ethyl type).[1]
Collection Risks
- Hydrolysis degradation can occur in alkaline conditions (glass surfaces).[1], [2]
- Cyanoacrylates undergo ultraviolet degradation.[1], [2]
Physical and Chemical Properties
- Uncured glue soluble in acetone, methanol, toluene, MEK.
- Cured glue is slightly soluble in DMF or nitromethane.
- Soaking in acetone may decrease adhesion and cause the join to separate.
- CAS = 7085-85-0
- Density = 1.05-1.10
Working Properties
Cures quickly
Forms/Sizes
Often sold in small container with application device
Comparisons
Physical Properties for Selected Thermoplastic Resins
General Characteristics of Polymers
Resources and Citations
- ↑ 1.0 1.1 1.2 Jane L. Down and Elzbieta Kaminska, "A Preliminary Study of the Degradation of Cyanoacrylate Adhesives in the Presence and Absence of Fossil Material", Journal of Vertebrate Paleontology, Vol. 26, No. 3 (Sep. 11, 2006), pp. 519-525
- ↑ 2.0 2.1 A literature review of cyanoacrylate adhesives. Reviews in conservation no. 2 (2001), pp. 35-38 International Institute for Conservation of Historic and Artistic Works, London, United Kingdom
- Contributions: Kate Wight Tyler, AIC Plastics Panel, 2020.
- H.W.Coover, J.M.McIntire, "Cyanoacrylate Adhesives" in Handbook of Adhesives, I.Skeist (ed.), Van Nostrand Reinhold, New York, 1977, p.569-580.
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Irving Skeist, Handbook of Adhesives, Van Nostrand Reinhold Company, New York, 1977
- Wikipedia: http://en.wikipedia.org/wiki/Cyanoacrylate (Accessed Feb. 10, 2006)