Difference between revisions of "Zirconium chloride"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 9: | Line 9: | ||
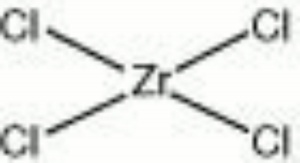
[[[SliderGallery rightalign|zirconium chloride.jpg~Chemical structure]]] | [[[SliderGallery rightalign|zirconium chloride.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| − | Soluble in water, ether. Decomposes in water forming hydrochloric acid and ZrOCl2. | + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/52782.htm MSDS] |
| + | |||
| + | == Physical and Chemical Properties == | ||
| + | |||
| + | * Soluble in water, ether. | ||
| + | * Decomposes in water forming hydrochloric acid and ZrOCl2. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 437 | + | | 437 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.803 | + | | 2.803 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 36: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 331 (sublimes) | + | | 331 C (sublimes) |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10307 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10307 | ||
Latest revision as of 12:13, 2 June 2022
Description
Hygroscopic zirconium salt that is used as a reagent in the preparation of silicone plastics and some water-repellent treatments for textiles. White, water-resistant leathers are produced using zirconium chloride and zirconium sulfate as tanning agents.
Synonyms and Related Terms
zirconium tetrachloride; zirconium (IV) chloride
Risks
- Fisher Scientific: MSDS
Physical and Chemical Properties
- Soluble in water, ether.
- Decomposes in water forming hydrochloric acid and ZrOCl2.
| Composition | ZrCl4 |
|---|---|
| CAS | 10026-11-6 |
| Melting Point | 437 C |
| Density | 2.803 g/ml |
| Molecular Weight | mol. wt. = 233.03 |
| Boiling Point | 331 C (sublimes) |
Resources and Citations
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10307