Difference between revisions of "Silk"
(username removed) |
|||
| (21 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File: | + | [[File:Italian dress 77.6.jpg|thumb|Court Dress<br>MFA Acc. #: 77.6]] |
== Description == | == Description == | ||
| + | [[File:25.550-CR7043-d1.jpg|thumb|Silk Patola<br>MFA Acc. #: 25.550]] | ||
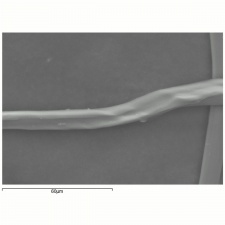
| + | [[File:Silk 200x CP.POL.jpg|thumb|Silk fibers at 200x]] | ||
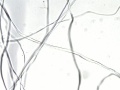
| + | A fine, lustrous natural fiber obtained from the catepillar cocoons of silk moths, such as the domesticated Bombyx mori. Silk moths are native to South and Southeast Asia as well as Northern China (e.g., Antheraea pernyi). According to legend, silk was discovered by Chinese Empress Si-Ling-Shi when a cocoon fell in her tea. China maintained a monopoly on the production of silk fabric for almost 3000 years. The worms were first cultivated in Japan about 195 CE and in Europe about 555 CE. Silk fibers contain a fibroin protein that can be decomposed with acid to form a mixture of amino acids: glycine (41.2 %), alanine (33.0%), serine (16.0 %), and tyrosine (11.4%) (Cook 1984). Microscopically, raw silk appears as two strands that are held together with sericin protein. The [[sericin|sericin]], or gum, is removed by boiling the fibers in soapy water or a dilute alkali. Longitudinal striations appear in the fibers due to wear. Silk readily absorbs soluble salts and is often treated with metal salts, such as tin phosphate/ sodium silicate or tin chloride, to increase its density. A moderately weighted silk contains about 25-50% salt. Weighted silks degrade more rapidly. | ||
| + | * For silk fiber identification, see http://cameo.mfa.org/wiki/Category:FRIL:_Silk | ||
| + | See also [[wild%20silk|wild silk]]. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
si (Chin.); soie (Fr.); silke (Dan., Sven.); Seide (Deut.); seda (Esp., Port.); zijde (Ned.); jedwab (Pol.); | si (Chin.); soie (Fr.); silke (Dan., Sven.); Seide (Deut.); seda (Esp., Port.); zijde (Ned.); jedwab (Pol.); | ||
| − | |||
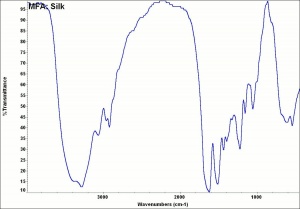
[[[SliderGallery rightalign|MFA- Silk.jpg~FTIR|silk370m.jpg~SEM|silk1000m.jpg~SEM]]] | [[[SliderGallery rightalign|MFA- Silk.jpg~FTIR|silk370m.jpg~SEM|silk1000m.jpg~SEM]]] | ||
| − | + | == Risks == | |
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Degraded by sunlight. Decomposed by strong acids. Resistant to moths, bacteria and fungi. Susceptible to carpet beetles. | Degraded by sunlight. Decomposed by strong acids. Resistant to moths, bacteria and fungi. Susceptible to carpet beetles. | ||
| + | == Physical and Chemical Properties == | ||
| − | + | * Soluble in hot, strong alkali. Damaged by weak alkali solutions (soap). Unaffected by most organic solvents. | |
| + | * Silk burns readily with an unsteady flame. It smells like burnt hair. The ash is readily crumbled. | ||
| − | + | For ''Bombyx mori'': | |
| + | * Cross section is triangular. | ||
| + | * Filament length = 250-750 meters long. | ||
| + | * Moisture regain = 11%; | ||
| + | * Elongation = 20-25% (normal) and 30% (wet); | ||
| + | * Melting Point = 175 C (dec) | ||
| + | * Density = 1.25-1.34 g/ml | ||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:Properties of Natural Fibers.pdf|Properties of Natural Fibers]] |
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 52: | Line 39: | ||
File:silk_seracin_lustre1.jpg|Seracin silk with lustre | File:silk_seracin_lustre1.jpg|Seracin silk with lustre | ||
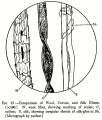
File:00000098B.jpg|Comparison of wool, cotton and silk fibers | File:00000098B.jpg|Comparison of wool, cotton and silk fibers | ||
| − | File:41 silk cult 200X pol.jpg|Cultivated silk | + | File:41 silk cult 200X pol.jpg|Cultivated silk at 200x polarized light |
| − | File:41 silk cult 200X.jpg|Cultivated silk | + | File:41 silk cult 200X.jpg|Cultivated silk at 200x |
| − | File:Irradiated silk_200X.jpg|Irradiated silk fiber | + | File:Irradiated silk_200X.jpg|Irradiated silk fiber at 200x |
File:wildsilkfiberslarge.jpg|Silk | File:wildsilkfiberslarge.jpg|Silk | ||
File:china silk.jpg|China silk | File:china silk.jpg|China silk | ||
| Line 60: | Line 47: | ||
</gallery> | </gallery> | ||
| + | == Resources and Citations == | ||
| − | + | * G.Cook, ''Handbook of Textile Fibres:I. Natural Fibres'', 5th edition, Merrow Publishing Co., Durham, England, 1984. | |
| − | + | * M.Brooks, S.O'Conner, J.McDonnell "The Application of Low-energy X-radiography in the Examination and Investigation of Degraded Historic Silk Textiles" ICOM Preprints, Edinburgh, Vol. II, p.670-79, 1996. | |
| − | * | + | * Hoechst Celanese Corporation, ''Dictionary of Fiber & Textile Technology'' (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990 |
| − | + | * S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 | |
| − | * | + | * Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 |
| − | |||
| − | * | ||
| − | |||
| − | |||
| − | |||
* ''Identification of Textile Materials'', The Textile Institute, Manchester, England, 1985 | * ''Identification of Textile Materials'', The Textile Institute, Manchester, England, 1985 | ||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "silk" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "silk" | ||
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "silk" [Accessed November 7, 2001]. . | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "silk" | + | * Website: www.fabrics.net |
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Silk Silk (Accessed Nov. 29, 2005 and March 2025) | |
| − | * Website | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 721 | |
| − | * Wikipedia | ||
| − | |||
| − | * | ||
| − | |||
| − | * | ||
| − | |||
* ''Encyclopedia of Archaeology'', Glyn E. Daniel, ed., Thomas Y. Crowell Co., New York, 1977 | * ''Encyclopedia of Archaeology'', Glyn E. Daniel, ed., Thomas Y. Crowell Co., New York, 1977 | ||
| − | + | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | |
| − | * | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:00, 23 March 2025
Description
A fine, lustrous natural fiber obtained from the catepillar cocoons of silk moths, such as the domesticated Bombyx mori. Silk moths are native to South and Southeast Asia as well as Northern China (e.g., Antheraea pernyi). According to legend, silk was discovered by Chinese Empress Si-Ling-Shi when a cocoon fell in her tea. China maintained a monopoly on the production of silk fabric for almost 3000 years. The worms were first cultivated in Japan about 195 CE and in Europe about 555 CE. Silk fibers contain a fibroin protein that can be decomposed with acid to form a mixture of amino acids: glycine (41.2 %), alanine (33.0%), serine (16.0 %), and tyrosine (11.4%) (Cook 1984). Microscopically, raw silk appears as two strands that are held together with sericin protein. The Sericin, or gum, is removed by boiling the fibers in soapy water or a dilute alkali. Longitudinal striations appear in the fibers due to wear. Silk readily absorbs soluble salts and is often treated with metal salts, such as tin phosphate/ sodium silicate or tin chloride, to increase its density. A moderately weighted silk contains about 25-50% salt. Weighted silks degrade more rapidly.
- For silk fiber identification, see http://cameo.mfa.org/wiki/Category:FRIL:_Silk
See also Wild silk.
Synonyms and Related Terms
si (Chin.); soie (Fr.); silke (Dan., Sven.); Seide (Deut.); seda (Esp., Port.); zijde (Ned.); jedwab (Pol.);
Risks
Degraded by sunlight. Decomposed by strong acids. Resistant to moths, bacteria and fungi. Susceptible to carpet beetles.
Physical and Chemical Properties
- Soluble in hot, strong alkali. Damaged by weak alkali solutions (soap). Unaffected by most organic solvents.
- Silk burns readily with an unsteady flame. It smells like burnt hair. The ash is readily crumbled.
For Bombyx mori:
- Cross section is triangular.
- Filament length = 250-750 meters long.
- Moisture regain = 11%;
- Elongation = 20-25% (normal) and 30% (wet);
- Melting Point = 175 C (dec)
- Density = 1.25-1.34 g/ml
Comparisons
Additional Images
Resources and Citations
- G.Cook, Handbook of Textile Fibres:I. Natural Fibres, 5th edition, Merrow Publishing Co., Durham, England, 1984.
- M.Brooks, S.O'Conner, J.McDonnell "The Application of Low-energy X-radiography in the Examination and Investigation of Degraded Historic Silk Textiles" ICOM Preprints, Edinburgh, Vol. II, p.670-79, 1996.
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- Identification of Textile Materials, The Textile Institute, Manchester, England, 1985
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "silk"
- Encyclopedia Britannica, http://www.britannica.com Comment: "silk" [Accessed November 7, 2001]. .
- Website: www.fabrics.net
- Wikipedia: [https://en.wikipedia.org/wiki/Silk Silk (Accessed Nov. 29, 2005 and March 2025)
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 721
- Encyclopedia of Archaeology, Glyn E. Daniel, ed., Thomas Y. Crowell Co., New York, 1977
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986