Difference between revisions of "Dioctyl phthalate"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A light-color liquid commonly called bis(2-ethylhexyl)phthalate. Dioctyl phthalate is used as a [ | + | A light-color liquid commonly called bis(2-ethylhexyl)phthalate. Dioctyl phthalate is used as a [[plasticizer]] in [[cellulose acetate]], [[cellulose acetate butyrate]], [[polystyrene]], [[vinyl chloride]], and [[Polyvinyl chloride acetate|vinyl chloride acetate]] polymers. Polymers may contain DOP in concentrations of 1-40%. The European Commission has banned the use of many phthalates, including DOP, in PVC toys. |
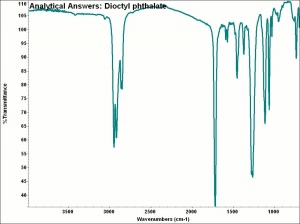
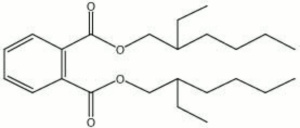
| − | + | [[[SliderGallery rightalign|aaiDOP.jpg~FTIR|dioctyl phthalate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
DOP; DEHP; di(2-ethylhexyl) phthalate; bis (2-ethylhexyl) phthalate; di-2-ethyl hexyl phthalate | DOP; DEHP; di(2-ethylhexyl) phthalate; bis (2-ethylhexyl) phthalate; di-2-ethyl hexyl phthalate | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Suspected carcinogen. | ||
| + | * Irritant to eyes, nose, lungs, and skin. | ||
| + | * Combustible. Flash point = 215 C | ||
| + | * Millipore Sigma: [https://www.sigmaaldrich.com/US/en/product/aldrich/d201154 SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Miscible in mineral oil. Insoluble in water. | Miscible in mineral oil. Insoluble in water. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -50 | + | | -50 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.9861 | + | | 0.9861 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 36: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 231 | + | | 231 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1291 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1291 | ||
Latest revision as of 15:49, 21 July 2022
Description
A light-color liquid commonly called bis(2-ethylhexyl)phthalate. Dioctyl phthalate is used as a Plasticizer in Cellulose acetate, Cellulose acetate butyrate, Polystyrene, Vinyl chloride, and vinyl chloride acetate polymers. Polymers may contain DOP in concentrations of 1-40%. The European Commission has banned the use of many phthalates, including DOP, in PVC toys.
Synonyms and Related Terms
DOP; DEHP; di(2-ethylhexyl) phthalate; bis (2-ethylhexyl) phthalate; di-2-ethyl hexyl phthalate
Risks
- Suspected carcinogen.
- Irritant to eyes, nose, lungs, and skin.
- Combustible. Flash point = 215 C
- Millipore Sigma: SDS
Physical and Chemical Properties
Miscible in mineral oil. Insoluble in water.
| Composition | C24H38O4 |
|---|---|
| CAS | 117-81-7 |
| Melting Point | -50 C |
| Density | 0.9861 g/ml |
| Molecular Weight | mol. wt. = 390.5 |
| Boiling Point | 231 C |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1291