Difference between revisions of "Dimethylhydrazine"
Jump to navigation
Jump to search
(username removed) |
(→Risks) |
||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
A colorless, flammable, hygroscopic liquid that reacts with water and moisture to produce heat. The principal use of dimethylhydrazine is as the base in rocket fuel formulations, but it is also found in some photographic developing solutions. | A colorless, flammable, hygroscopic liquid that reacts with water and moisture to produce heat. The principal use of dimethylhydrazine is as the base in rocket fuel formulations, but it is also found in some photographic developing solutions. | ||
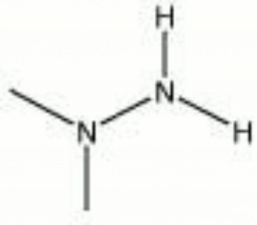
| − | + | [[[SliderGallery rightalign|dimethylhydrazine.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
1,1-dimethylhydrazine; n,n-dimethylhydrazine | 1,1-dimethylhydrazine; n,n-dimethylhydrazine | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Toxic by ingestion, inhalation and skin absorption. | ||
| + | * Suspected carcinogen. | ||
| + | * Exposure may cause skin irritation, choking, nausea, convulsions and liver injury. | ||
| + | * Highly flammable. Flash point = -15C. Vapors are explosive. | ||
| + | * Decomposes to produce toxic gases. | ||
| + | * Millipore Sigma: [https://www.sigmaaldrich.com/US/en/product/aldrich/d161608 SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water and ethanol. | Soluble in water and ethanol. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -58 | + | | -58 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.782 | + | | 0.782 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 63 | + | | 63 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3297 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:12, 21 July 2022
Description
A colorless, flammable, hygroscopic liquid that reacts with water and moisture to produce heat. The principal use of dimethylhydrazine is as the base in rocket fuel formulations, but it is also found in some photographic developing solutions.
Synonyms and Related Terms
1,1-dimethylhydrazine; n,n-dimethylhydrazine
Risks
- Toxic by ingestion, inhalation and skin absorption.
- Suspected carcinogen.
- Exposure may cause skin irritation, choking, nausea, convulsions and liver injury.
- Highly flammable. Flash point = -15C. Vapors are explosive.
- Decomposes to produce toxic gases.
- Millipore Sigma: SDS
Physical and Chemical Properties
Soluble in water and ethanol.
| Composition | (CH3)2NNH2 |
|---|---|
| CAS | 57-14-7 |
| Melting Point | -58 C |
| Density | 0.782 g/ml |
| Molecular Weight | mol. wt. = 60.1 |
| Boiling Point | 63 C |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3297
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993