Difference between revisions of "Potassium phosphate"
Jump to navigation
Jump to search
(username removed) |
(→Risks) |
||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
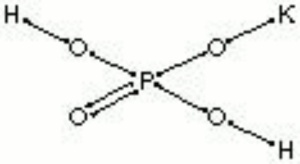
| − | White granular crystals. Potassium phosphate monobasic is used as a [ | + | White granular crystals. Potassium phosphate monobasic is used as a [[buffer|buffering agent]]. |
| − | + | [[[SliderGallery rightalign|potassium phosphate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
potassium phosphate monobasic; potassium dihydrogen phosphate | potassium phosphate monobasic; potassium dihydrogen phosphate | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Contact or inhalation may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=P285500&productDescription=POT+PHOSPHATE+MONO+CR+ACS+500G&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water. pH = 4.4-4.7 | Soluble in water. pH = 4.4-4.7 | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 253 | + | | 253 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.34 | + | | 2.34 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 34: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 400 (dec) | + | | 400 C (dec) |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7542 |
| − | * | + | * Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, ''Notes for Workshop on New Methods in the Cleaning of Paintings'', J.Paul Getty Trust, Los Angeles, 1990 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 10:36, 25 August 2022
Description
White granular crystals. Potassium phosphate monobasic is used as a buffering agent.
Synonyms and Related Terms
potassium phosphate monobasic; potassium dihydrogen phosphate
Risks
- Contact or inhalation may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water. pH = 4.4-4.7
| Composition | KH2PO4 |
|---|---|
| CAS | 7778-77-0 |
| Melting Point | 253 C |
| Density | 2.34 g/ml |
| Molecular Weight | mol. wt. = 136.09 |
| Boiling Point | 400 C (dec) |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7542
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990