Difference between revisions of "Tetrachloroethylene"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 9: | Line 9: | ||
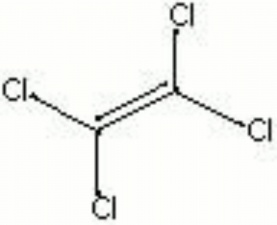
[[[SliderGallery rightalign|tetrachloroethylene.jpg~Chemical structure]]] | [[[SliderGallery rightalign|tetrachloroethylene.jpg~Chemical structure]]] | ||
| − | == | + | == Physical and Chemical Properties == |
Miscible in ethanol, ether, chloroform, benzene. Insoluble in water. | Miscible in ethanol, ether, chloroform, benzene. Insoluble in water. | ||
| Line 22: | Line 22: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 22 | + | | 22 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.6230 | + | | 1.6230 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 34: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 121 | + | | 121 C |
|} | |} | ||
| − | == | + | == Risks == |
| − | Irritating to eyes and skin. Potential carcinogen. Dangerous to the environment. | + | * Irritating to eyes and skin. |
| + | * Potential carcinogen. | ||
| + | * Dangerous to the environment. | ||
| + | * Nonflammable, but may decompose in the presence of flames or UV light to form toxic fumes (phosgene, hydrogen chloride). | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC138015000&productDescription=TETRACHLOROETHYLENE+99%25+500ML&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| − | + | ==Resources and Citations== | |
| − | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | |
| − | + | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | |
| − | * | + | * Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9332; ref. index=1.5055 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9332; ref. index=1.5055 | ||
Latest revision as of 10:54, 8 June 2022
Description
Colorless, chlorinated hydrocarbon with an ether-like odor. Tetrachloroethylene (TCE) was first prepared by Faraday in 1921. It is currently used as a dry-cleaning solvent and as a vapor-degreaser for metals. TCE is a suspected carcinogen.
Synonyms and Related Terms
TCE; tetrachloroethene; ethylene tetrachloride; perchloroethylene; Perclene; Vaclene [DuPont]
Physical and Chemical Properties
Miscible in ethanol, ether, chloroform, benzene. Insoluble in water.
| Composition | Cl2C:CCl2 |
|---|---|
| CAS | 127-18-4 |
| Melting Point | 22 C |
| Density | 1.6230 g/ml |
| Molecular Weight | mol. wt.= 165.83 . |
| Refractive Index | 1.5055 |
| Boiling Point | 121 C |
Risks
- Irritating to eyes and skin.
- Potential carcinogen.
- Dangerous to the environment.
- Nonflammable, but may decompose in the presence of flames or UV light to form toxic fumes (phosgene, hydrogen chloride).
- ThermoFisher: SDS
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9332; ref. index=1.5055
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.504