Difference between revisions of "Vanadium tetrachloride"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Reddish-brown liquid. Vanadium tetrachloride is used in glassmaking to produce [ | + | Reddish-brown liquid. Vanadium tetrachloride is used in glassmaking to produce [[iridescence|iridescence]]. |
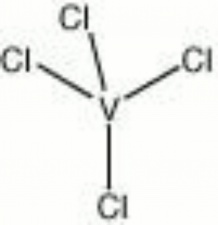
| − | + | [[[SliderGallery rightalign|vanadium tetrachloride.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
vanadium (IV) chloride | vanadium (IV) chloride | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Toxic by inhalation, ingestion and skin absorption. | ||
| + | * Corrosive on contact. | ||
| + | * Nonflammable. | ||
| + | * Reacts explosively with water or moisture. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/95621.htm MSDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in absolute alcohol. | Soluble in absolute alcohol. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -28 | + | | -28 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.816 | + | | 1.816 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 148-154 | + | | 148-154 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Latest revision as of 13:20, 23 June 2022
Description
Reddish-brown liquid. Vanadium tetrachloride is used in glassmaking to produce Iridescence.
Synonyms and Related Terms
vanadium (IV) chloride
Risks
- Toxic by inhalation, ingestion and skin absorption.
- Corrosive on contact.
- Nonflammable.
- Reacts explosively with water or moisture.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in absolute alcohol.
| Composition | VCl4 |
|---|---|
| CAS | 7632-51-1 |
| Melting Point | -28 C |
| Density | 1.816 g/ml |
| Molecular Weight | mol. wt. = 192.75 |
| Boiling Point | 148-154 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976