Difference between revisions of "Fluorite"
(username removed) |
|||
| (13 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:Fluorite and ivory SC326257.jpg|thumb|Amulet with ivory and fluorite; MFA # 13.3503]] | ||
| + | == Description == | ||
[[File:Fluoritedw.jpg|thumb|Fluorite crystals]] | [[File:Fluoritedw.jpg|thumb|Fluorite crystals]] | ||
| − | + | A crystalline mineral composed of [[calcium fluoride]]. Fluorite, or fluorspar, can occur as large transparent or translucent crystals with a glassy luster. The crystals may be white, green, pink, blue, purple, yellow or brown but all exhibit a strong autofluorescence in ultraviolet light. Calcium fluorite is inherently colorless and the color is thought to results from defects in the cystal lattice generated by impurities. Fluorite is found throughout the world with significant deposits in China, Mongolia, South Africa, Russia, France, Germany, Austria, Switzerland, Norway, England, Canada (Ontario), Mexico, and the United States (New Mexico, Arizona, Colorado, Oklahoma, Missouri, Illinois, Kentucky, Ohio, New Hampshire, New York.). Fluorite has been gathered or mined since Neolithic times and used for small carved items, beads, and gemstones. Blue John, a blue-purple fluorite found in Derbyshire County, England, was carved for ornamental vases and boxes. Fluorite is the primary source for fluorine. Because it melts easily, powdered fluorite is used as flux in metallurgy. Fluorite is also used as an opacifier in opalescent glass. Small amounts of fluorite in pottery glazes and ceramic enamels produce a transparent green color but toxic gases may be released during firing. Optical quality fluorite is used for apochromatic lenses because it has a low refractive index and low dispersion. Purple fluorite has been used as a pigment, particularly where good local deposits are known, such as in Southern Germany and the Tyrol. Indeed almost all published occurrences are from panel paintings, polychrome sculpture and wall paintings from this area in the period 1470-1520. Exceptions include a number of early 16th century Netherlandish paintings (Spring 2000). | |
| − | |||
| − | A crystalline mineral composed of [ | ||
| − | |||
[[File:fluorite2dw.jpg|thumb|Fluorite crystals]] | [[File:fluorite2dw.jpg|thumb|Fluorite crystals]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
calcium fluoride; fluorspar; fluor; fluospar; Derbyshire spar; Blue John; bluejohn; fluate; Fluorit (Deut.); Flussspat (Deut.); fluorita (Esp.); fluorite (Fr., Port.); vloeispaat (Ned.); fluoriet (Ned.); fluoryt (Pol.) | calcium fluoride; fluorspar; fluor; fluospar; Derbyshire spar; Blue John; bluejohn; fluate; Fluorit (Deut.); Flussspat (Deut.); fluorita (Esp.); fluorite (Fr., Port.); vloeispaat (Ned.); fluoriet (Ned.); fluoryt (Pol.) | ||
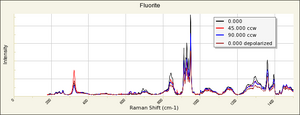
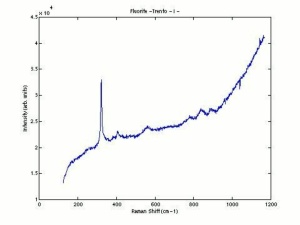
| + | [[[SliderGallery rightalign|Fluorite Raman RRUFF R060548.png~Raman (RRUFF)|Fluoriteitaly2.jpg~Raman (U of Parma)]]] | ||
| + | == Risks == | ||
| − | [ | + | * Fluorite reacts with silica at high temperatures to produce toxic silicon tetrafluoride gas. |
| − | + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=C89250&productDescription=CALCIUM+FLUORIDE+CERT+250GM&vendorId=VN00033897&countryCode=US&language=en SDS] | |
| − | == | + | == Physical and Chemical Properties == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | Pale of colorless under plane-polarized light | + | * Isometric crystal system with coarse crystals (cubic, fibrous, granular, massive) |
| + | * Perfect octahedral cleavage in four directions | ||
| + | * Fracture = uneven to subconchoidal | ||
| + | * Luster = vitreous to subvitreous | ||
| + | * Streak = white | ||
| + | * Slightly soluble in water | ||
| + | * Pale of colorless under plane-polarized light; isotropic | ||
| + | * Fluorescence = may be fluorescent, phosphorescent, thermoluminescent and/or triboluminescent | ||
| + | * Birefringence = none | ||
| + | * Inclusions = triangular or tetrahedral negative crystals; liquid inclusions; strong color zoning | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 28: | Line 34: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 1350 | + | | 1350 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.18 | + | | 3.18 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.432-1.437 | | 1.432-1.437 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 49: | Line 47: | ||
[[media:download_file_457.pdf|Properties of Common Gemstones]] | [[media:download_file_457.pdf|Properties of Common Gemstones]] | ||
| − | + | ==Resources and Citations== | |
| − | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | |
| − | == | + | * M. Spring, ‘Occurrences of the purple pigment fluorite on paintings in the National Gallery’, National Gallery Technical Bulletin 21, 2000, 20-27. |
| − | + | * M. Richter and R. Fuchs, ‘Violetter Flußspat’, in ''Restauro''1997, 316-23. | |
| − | * | + | * Mineralogy Database: [http://www.webmineral.com/data/Fluorite.shtml Fluorite] Record content reviewed by EU-Artech November 2007. |
| − | + | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Vol. 2, page 5. n=1.433-1.543 | |
| − | * | + | * C.S. Hurlbut, ''Dana's Manual of Mineralogy'', John Wiley & Sons, New York, London, 18th ed. (reprinted from 1966), 1971 |
| − | + | * Helen Howard, Contributed information, November 2007. | |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | + | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | |
| − | * | + | * Robert Fournier, ''Illustrated Dictionary of Practical Pottery'', Chilton Book Company, Radnor, PA, 1992 |
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "fluorite" [Accessed December 11, 2001] (BW photo) | |
| − | * | + | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 |
| − | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | |
| − | * | + | * Sue Fuller, ''Rocks and Minerals'', DK Publishing, Inc., New York City, 1995 |
| − | + | * J.Gordon Cook, ''Handbook of Textile Fibres:I Natural Fibres'', Merrow Publishing Co. , Durham, England, 1984 | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "fluorite" | + | * Wikipedia: [https://en.wikipedia.org/wiki/Fluorite Fluorite] (Accessed Sept. 7, 2005 and Dec 2022) |
| − | |||
| − | * | ||
| − | |||
| − | * | ||
| − | |||
| − | * | ||
| − | |||
| − | * | ||
| − | |||
| − | * Wikipedia | ||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=3.18 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=3.18 | ||
| − | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | |
| − | * | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:23, 4 January 2023
Description
A crystalline mineral composed of Calcium fluoride. Fluorite, or fluorspar, can occur as large transparent or translucent crystals with a glassy luster. The crystals may be white, green, pink, blue, purple, yellow or brown but all exhibit a strong autofluorescence in ultraviolet light. Calcium fluorite is inherently colorless and the color is thought to results from defects in the cystal lattice generated by impurities. Fluorite is found throughout the world with significant deposits in China, Mongolia, South Africa, Russia, France, Germany, Austria, Switzerland, Norway, England, Canada (Ontario), Mexico, and the United States (New Mexico, Arizona, Colorado, Oklahoma, Missouri, Illinois, Kentucky, Ohio, New Hampshire, New York.). Fluorite has been gathered or mined since Neolithic times and used for small carved items, beads, and gemstones. Blue John, a blue-purple fluorite found in Derbyshire County, England, was carved for ornamental vases and boxes. Fluorite is the primary source for fluorine. Because it melts easily, powdered fluorite is used as flux in metallurgy. Fluorite is also used as an opacifier in opalescent glass. Small amounts of fluorite in pottery glazes and ceramic enamels produce a transparent green color but toxic gases may be released during firing. Optical quality fluorite is used for apochromatic lenses because it has a low refractive index and low dispersion. Purple fluorite has been used as a pigment, particularly where good local deposits are known, such as in Southern Germany and the Tyrol. Indeed almost all published occurrences are from panel paintings, polychrome sculpture and wall paintings from this area in the period 1470-1520. Exceptions include a number of early 16th century Netherlandish paintings (Spring 2000).
Synonyms and Related Terms
calcium fluoride; fluorspar; fluor; fluospar; Derbyshire spar; Blue John; bluejohn; fluate; Fluorit (Deut.); Flussspat (Deut.); fluorita (Esp.); fluorite (Fr., Port.); vloeispaat (Ned.); fluoriet (Ned.); fluoryt (Pol.)
Risks
- Fluorite reacts with silica at high temperatures to produce toxic silicon tetrafluoride gas.
- ThermoFisher: SDS
Physical and Chemical Properties
- Isometric crystal system with coarse crystals (cubic, fibrous, granular, massive)
- Perfect octahedral cleavage in four directions
- Fracture = uneven to subconchoidal
- Luster = vitreous to subvitreous
- Streak = white
- Slightly soluble in water
- Pale of colorless under plane-polarized light; isotropic
- Fluorescence = may be fluorescent, phosphorescent, thermoluminescent and/or triboluminescent
- Birefringence = none
- Inclusions = triangular or tetrahedral negative crystals; liquid inclusions; strong color zoning
| Composition | CaF2 |
|---|---|
| Mohs Hardness | 4.0 |
| Melting Point | 1350 C |
| Density | 3.18 g/ml |
| Refractive Index | 1.432-1.437 |
Comparisons
Properties of Common Gemstones
Resources and Citations
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- M. Spring, ‘Occurrences of the purple pigment fluorite on paintings in the National Gallery’, National Gallery Technical Bulletin 21, 2000, 20-27.
- M. Richter and R. Fuchs, ‘Violetter Flußspat’, in Restauro1997, 316-23.
- Mineralogy Database: Fluorite Record content reviewed by EU-Artech November 2007.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Vol. 2, page 5. n=1.433-1.543
- C.S. Hurlbut, Dana's Manual of Mineralogy, John Wiley & Sons, New York, London, 18th ed. (reprinted from 1966), 1971
- Helen Howard, Contributed information, November 2007.
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Robert Fournier, Illustrated Dictionary of Practical Pottery, Chilton Book Company, Radnor, PA, 1992
- Encyclopedia Britannica, http://www.britannica.com Comment: "fluorite" [Accessed December 11, 2001] (BW photo)
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Sue Fuller, Rocks and Minerals, DK Publishing, Inc., New York City, 1995
- J.Gordon Cook, Handbook of Textile Fibres:I Natural Fibres, Merrow Publishing Co. , Durham, England, 1984
- Wikipedia: Fluorite (Accessed Sept. 7, 2005 and Dec 2022)
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=3.18
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971