Difference between revisions of "Cobalt violet, light"
(username removed) |
|||
| (6 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A pale to medium violet pigment originally composed of [ | + | A pale to medium violet pigment originally composed of [[cobaltous arsenate]]. Cobaltous arsenate occurs in nature as cobalt bloom or [[erythrite]]. Once it was synthetically produced in 1880, it became an important permanent, violet pigment. Cobaltous arsenate is now rarely used because of its toxicity. It has been replaced by the use of [[cobaltous phosphate]] (deep cobalt violet) and [[cobaltous ammonium phosphate]]. Light cobalt violet was used as a colorant in paints, glass, glazes, and enamels. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
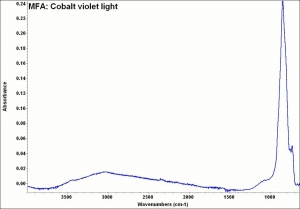
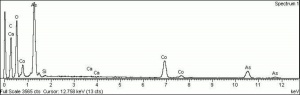
[[[SliderGallery rightalign|MFA- Cobalt violet light.jpg~FTIR|f501sem.jpg~SEM|f501edsbw.jpg~EDS]]] | [[[SliderGallery rightalign|MFA- Cobalt violet light.jpg~FTIR|f501sem.jpg~SEM|f501edsbw.jpg~EDS]]] | ||
| + | == Risks == | ||
| − | + | * Skin contact may cause allergies, especially on elbows, neck and ankles. | |
| + | * Chronic inhalation may cause asthma. | ||
| + | * Ingestion may cause vomiting, diarrhea and the sensation of hotness. | ||
| − | Monoclinic crystal system with prismatic or euhedral crystals. Perfect cleavage parallel to long axes. Weakly pleochroic. High birefringence under crossed polars. | + | ==Physical and Chemical Properties== |
| + | |||
| + | * Monoclinic crystal system with prismatic or euhedral crystals. | ||
| + | * Perfect cleavage parallel to long axes. | ||
| + | * Weakly pleochroic. | ||
| + | * High birefringence under crossed polars. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 23: | Line 31: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.06 | + | | 3.06 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 29: | Line 37: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | Pigments Through the Ages: [http://webexhibits.org/pigments/indiv/overview/coviolet.html Cobalt violet] | + | * Pigments Through the Ages: [http://webexhibits.org/pigments/indiv/overview/coviolet.html Cobalt violet] |
| − | + | * Corbeil, Marie-Claude, Jean-Pierre Charland, Elizabeth Moffatt. 'The characterization of cobalt violet pigments' ''Studies in Conservation'' vol.47 (2002), pp.237-249. | |
| − | * | + | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 |
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:11, 30 May 2022
Description
A pale to medium violet pigment originally composed of Cobaltous arsenate. Cobaltous arsenate occurs in nature as cobalt bloom or Erythrite. Once it was synthetically produced in 1880, it became an important permanent, violet pigment. Cobaltous arsenate is now rarely used because of its toxicity. It has been replaced by the use of Cobaltous phosphate (deep cobalt violet) and Cobaltous ammonium phosphate. Light cobalt violet was used as a colorant in paints, glass, glazes, and enamels.
Synonyms and Related Terms
cobalt arsenate (arsenite); Pigment Violet 14; CI 77350; erythrite (mineral); violeta de cobalto claro (Esp.); Kobaltviolett (hell) (Deut.); violet de cobalt (clair) (Fr.); iodes toy kobaltioy anoikto (Gr.); violetto di cobalto chiaro (It.); cobalt violet (licht) (Ned.); violeta de cobalto, claro (Port.); red cobalt; cobalt bloom; ; pale cobalt violet
Risks
- Skin contact may cause allergies, especially on elbows, neck and ankles.
- Chronic inhalation may cause asthma.
- Ingestion may cause vomiting, diarrhea and the sensation of hotness.
Physical and Chemical Properties
- Monoclinic crystal system with prismatic or euhedral crystals.
- Perfect cleavage parallel to long axes.
- Weakly pleochroic.
- High birefringence under crossed polars.
| Composition | Co3(AsO4)2 - 8H2O |
|---|---|
| Mohs Hardness | 1.5-2.5 (erythrite) |
| Density | 3.06 g/ml |
| Refractive Index | 1.626-1.701 |
Resources and Citations
- Pigments Through the Ages: Cobalt violet
- Corbeil, Marie-Claude, Jean-Pierre Charland, Elizabeth Moffatt. 'The characterization of cobalt violet pigments' Studies in Conservation vol.47 (2002), pp.237-249.
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000