Difference between revisions of "Naphthol"
Jump to navigation
Jump to search
(username removed) |
|||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white crystalline powder with a faint phenolic odor. Naphthol is an aromatic compound that is primarily used as an [ | + | A white crystalline powder with a faint phenolic odor. Naphthol is an aromatic compound that is primarily used as an [[antioxidant|antioxidant]] in rubbers. It is stable in air but turns dark with exposure to light. Naphthol is also used as a color developer and as a stabilizer for direct dyes. |
| − | See also [ | + | See also [[naphthol%20pigment|naphthol pigment]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
beta-naphthol; CI Developer 5; CI 37500; napthol (sp); naftol (Esp.); naphthol (Fr.); 2-naphthol; 2-hydroxynaphthalene; 2-naphthalenol; isonaphthol | beta-naphthol; CI Developer 5; CI 37500; napthol (sp); naftol (Esp.); naphthol (Fr.); 2-naphthol; 2-hydroxynaphthalene; 2-naphthalenol; isonaphthol | ||
| − | |||
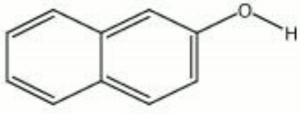
[[[SliderGallery rightalign|naphthol.jpg~Chemical structure]]] | [[[SliderGallery rightalign|naphthol.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| − | == | + | * Combustible. Flash point = 153C (307F) |
| + | * Skin irritant. Ingestion may cause vomiting, abdominal pain and convulsions. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/97240.htm MSDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in ethanol, ether, chloroform, glycerol, oils, alkaline solutions. Insoluble in water. | Soluble in ethanol, ether, chloroform, glycerol, oils, alkaline solutions. Insoluble in water. | ||
| Line 24: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 121-123 | + | | 121-123 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.22 | + | | 1.22 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 36: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 285-286 | + | | 285-286 C |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6471 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6471 | ||
| Line 56: | Line 51: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * | + | * B. Berrie, S.Q. Lomax, 'Azo Pigments: Their History, Synthesis, Properties and Use in Artists' Materials', ''Studies in the History of Art'' , National Gallery of Art, Washington DC, No. 57, 1997 |
| − | * Website | + | * Website: www.china-pigment.com - density =1.28; m.p.=123-124; b.p.=295 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 09:59, 19 October 2022
Description
A white crystalline powder with a faint phenolic odor. Naphthol is an aromatic compound that is primarily used as an Antioxidant in rubbers. It is stable in air but turns dark with exposure to light. Naphthol is also used as a color developer and as a stabilizer for direct dyes.
See also Naphthol pigment.
Synonyms and Related Terms
beta-naphthol; CI Developer 5; CI 37500; napthol (sp); naftol (Esp.); naphthol (Fr.); 2-naphthol; 2-hydroxynaphthalene; 2-naphthalenol; isonaphthol
Risks
- Combustible. Flash point = 153C (307F)
- Skin irritant. Ingestion may cause vomiting, abdominal pain and convulsions.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ethanol, ether, chloroform, glycerol, oils, alkaline solutions. Insoluble in water.
| Composition | C10H7OH |
|---|---|
| CAS | 135-19-3 |
| Melting Point | 121-123 C |
| Density | 1.22 g/ml |
| Molecular Weight | mol. wt.=144.17 |
| Boiling Point | 285-286 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6471
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- B. Berrie, S.Q. Lomax, 'Azo Pigments: Their History, Synthesis, Properties and Use in Artists' Materials', Studies in the History of Art , National Gallery of Art, Washington DC, No. 57, 1997
- Website: www.china-pigment.com - density =1.28; m.p.=123-124; b.p.=295