Difference between revisions of "Strontium sulfate"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | White crystals that occur in nature as the mineral [ | + | White crystals that occur in nature as the mineral [[celestite|celestite]]. Strontium sulfate was used occasionally as a white artist pigment in England in the early 19th century. It was soon replaced by [[barium%20sulfate|barium sulfate]]. Currently strontium sulfate is used as a red colorant in pyrotechnics, [[ceramic|ceramics]], and [[glass|glass]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | strontium white; strontium sulphate (Br.); sulfato de estroncio (Esp.); sulfate de strontium (Fr.); sulfato de | + | strontium white; strontium sulphate (Br.); sulfato de estroncio (Esp.); sulfate de strontium (Fr.); sulfato de estrôncio (Port.); celestite |
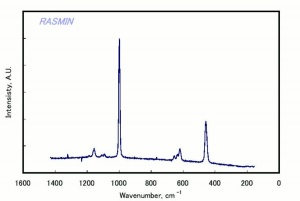
[[[SliderGallery rightalign|celestiteRS.jpg~Raman]]] | [[[SliderGallery rightalign|celestiteRS.jpg~Raman]]] | ||
| − | == | + | == Physical and Chemical Properties == |
Slightly soluble in concentrated acids and water. Insoluble in ethanol and dilute sulfuric acid. | Slightly soluble in concentrated acids and water. Insoluble in ethanol and dilute sulfuric acid. | ||
| Line 23: | Line 23: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 1605 | + | | 1605 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.71-3.97 | + | | 3.71-3.97 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 32: | Line 32: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9013 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9013 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Strontium_sulfate (Accessed Nov. 9, 2005) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:31, 6 June 2022
Description
White crystals that occur in nature as the mineral Celestite. Strontium sulfate was used occasionally as a white artist pigment in England in the early 19th century. It was soon replaced by Barium sulfate. Currently strontium sulfate is used as a red colorant in pyrotechnics, ceramics, and Glass.
Synonyms and Related Terms
strontium white; strontium sulphate (Br.); sulfato de estroncio (Esp.); sulfate de strontium (Fr.); sulfato de estrôncio (Port.); celestite
Physical and Chemical Properties
Slightly soluble in concentrated acids and water. Insoluble in ethanol and dilute sulfuric acid.
| Composition | SrSO4 |
|---|---|
| CAS | 7759-02-6 |
| Melting Point | 1605 C |
| Density | 3.71-3.97 g/ml |
| Molecular Weight | mol. wt. = 183.68 |
Resources and Citations
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9013
- Wikipedia: http://en.wikipedia.org/wiki/Strontium_sulfate (Accessed Nov. 9, 2005)