Difference between revisions of "Lanolin"
(username removed) |
|||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A semisolid waxy material that is purified from the grease, or [ | + | A semisolid waxy material that is purified from the grease, or [[degras]], extracted from [[wool]]. Lanolin is a complex mixture of 33 high molecular weight alcohols and 36 fatty acids, including [[cholesterol]]. It also contains about 5% free alcohols and free acids. One of the unique properties of lanolin is that it can incorporate up to 25-30% water without separation. Since it is readily absorbed by skin, lanolin is used as an emollient in [[leather]] dressings as well as for cosmetics and ointments. It is also used as a rust preventative. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 7: | ||
lanoline; animal wax; wool wax; wool grease; wool fat; lanoline; Lanain; Lanum; adeps lanae; oesipos; Agnolin; Lantrol; Loanilin (Deut., Sven.); Lanolina (Pol); | lanoline; animal wax; wool wax; wool grease; wool fat; lanoline; Lanain; Lanum; adeps lanae; oesipos; Agnolin; Lantrol; Loanilin (Deut., Sven.); Lanolina (Pol); | ||
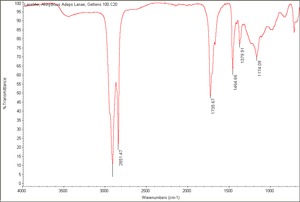
| − | == | + | [[[SliderGallery rightalign|Lanoline, Anhydrous Adeps Lanae, Gettens 100.C20.TIF~FTIR(MFA)]]] |
| + | == Risks == | ||
| + | |||
| + | Contact will cause of reaction in persons with wool allergies. | ||
| + | |||
| + | Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-l/S25376.pdf SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in benzene, chloroform, ether, carbon, acetone, ligroin, carbon disulfide and ethanol. Insoluble in water. | Soluble in benzene, chloroform, ether, carbon, acetone, ligroin, carbon disulfide and ethanol. Insoluble in water. | ||
| Line 16: | Line 22: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 36-43 | + | | 36-43 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.932-0.9457 | + | | 0.932-0.9457 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.478-1.482 | | 1.478-1.482 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_10.pdf|Properties of Natural Waxes]] |
| − | |||
| − | |||
| − | == | + | == Resources and Citations == |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 49: | Line 47: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5371 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5371 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Lanolin" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Lanolin" [Accessed 18 Oct. 2005]. |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Lanolin (Accessed Jan. 10, 2006) |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point = 36-43C, density=0.932-0.9457, ref. index = 1.478-1.482, iodine value=15.0-46.9, acid value=5.6-22.0, saponification value = 80-127 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point = 36-43C, density=0.932-0.9457, ref. index = 1.478-1.482, iodine value=15.0-46.9, acid value=5.6-22.0, saponification value = 80-127 | ||
Latest revision as of 17:02, 7 September 2022
Description
A semisolid waxy material that is purified from the grease, or Degras, extracted from Wool. Lanolin is a complex mixture of 33 high molecular weight alcohols and 36 fatty acids, including Cholesterol. It also contains about 5% free alcohols and free acids. One of the unique properties of lanolin is that it can incorporate up to 25-30% water without separation. Since it is readily absorbed by skin, lanolin is used as an emollient in Leather dressings as well as for cosmetics and ointments. It is also used as a rust preventative.
Synonyms and Related Terms
lanoline; animal wax; wool wax; wool grease; wool fat; lanoline; Lanain; Lanum; adeps lanae; oesipos; Agnolin; Lantrol; Loanilin (Deut., Sven.); Lanolina (Pol);
Risks
Contact will cause of reaction in persons with wool allergies.
Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in benzene, chloroform, ether, carbon, acetone, ligroin, carbon disulfide and ethanol. Insoluble in water.
Refined: iodine value=15.0-46.9, acid value=5.6-22.0, saponification value = 80-127
| Melting Point | 36-43 C |
|---|---|
| Density | 0.932-0.9457 g/ml |
| Refractive Index | 1.478-1.482 |
Comparisons
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5371
- Encyclopedia Britannica, http://www.britannica.com Comment: "Lanolin" [Accessed 18 Oct. 2005].
- Wikipedia: http://en.wikipedia.org/wiki/Lanolin (Accessed Jan. 10, 2006)
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point = 36-43C, density=0.932-0.9457, ref. index = 1.478-1.482, iodine value=15.0-46.9, acid value=5.6-22.0, saponification value = 80-127
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 880
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000