Difference between revisions of "Acetanilide"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A white, odorless powder that has a burning taste. Acetanilide is produced from aniline using [ | + | A white, odorless powder that has a burning taste. Acetanilide is produced from aniline using [[acetic%20acid|acetic acid]]. The shiny, leaflet crystals are stable in air. Acetanilide was used in the 19th century to treat fever and headaches but was discontinued because of toxic side effects. It is still used in the manufacture of medicines and dyes, as an accelerator for rubber [[vulcanization|vulcanization]], as a stabilizer for [[cellulose%20ester|cellulose ester]] dopes and lacquers, and as a synthetic [[camphor|camphor]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 10: | Line 10: | ||
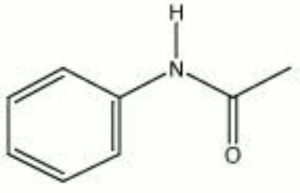
[[[SliderGallery rightalign|acetanilide.jpg~Chemical structure]]] | [[[SliderGallery rightalign|acetanilide.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Highly toxic by ingestion. | ||
| + | * Causes cyanosis. | ||
| + | * Skin contact and inhalation cause irritation. | ||
| + | * Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-a/S25117.pdf SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in hot water, ethanol, ether, chloroform, acetone, glycerol and benzene. | Soluble in hot water, ethanol, ether, chloroform, acetone, glycerol and benzene. | ||
| Line 23: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 114-116 | + | | 114-116 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.2105 | + | | 1.2105 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 32: | Line 39: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 303.8 | + | | 303.8 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 47: | Line 48: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Acetanilide (Accessed Oct. 18, 2005) |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 14:43, 19 April 2022
Description
A white, odorless powder that has a burning taste. Acetanilide is produced from aniline using Acetic acid. The shiny, leaflet crystals are stable in air. Acetanilide was used in the 19th century to treat fever and headaches but was discontinued because of toxic side effects. It is still used in the manufacture of medicines and dyes, as an accelerator for rubber Vulcanization, as a stabilizer for Cellulose ester dopes and lacquers, and as a synthetic Camphor.
Synonyms and Related Terms
n-phenylacetamide; antifebrin; acetylaniline; acetylaminobenzene; acetic acid anilide; acetanil; n-acetylaniline; n-phenyl acetamide
Risks
- Highly toxic by ingestion.
- Causes cyanosis.
- Skin contact and inhalation cause irritation.
- Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in hot water, ethanol, ether, chloroform, acetone, glycerol and benzene.
| Composition | C8H9NO |
|---|---|
| CAS | 103-84-4 |
| Melting Point | 114-116 C |
| Density | 1.2105 g/ml |
| Molecular Weight | mol. wt.=135.17 |
| Boiling Point | 303.8 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Wikipedia: http://en.wikipedia.org/wiki/Acetanilide (Accessed Oct. 18, 2005)
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998