Difference between revisions of "Salicylic acid"
Jump to navigation
Jump to search
(username removed) |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 2: | Line 2: | ||
A white crystalline powder. Salicylic acid occurs naturally in wintergreen leaves and the bark of sweet birch. It is prepared synthetically for used in the manufacture of aspirin and dyestuffs. Salicylic acid is also used as a preservative and fungicide in pastes. | A white crystalline powder. Salicylic acid occurs naturally in wintergreen leaves and the bark of sweet birch. It is prepared synthetically for used in the manufacture of aspirin and dyestuffs. Salicylic acid is also used as a preservative and fungicide in pastes. | ||
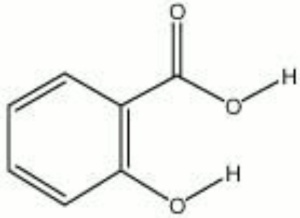
| − | + | [[[SliderGallery rightalign|salicylic acid.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
2-hydroxybenzoic acid; Keralyt; Occlusal; Verrugon; o-hydroxybenzoic acid | 2-hydroxybenzoic acid; Keralyt; Occlusal; Verrugon; o-hydroxybenzoic acid | ||
| − | + | == Risks == | |
| − | == | + | * Combustible. |
| + | * Toxic by ingestion of large amounts. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AAA1225336&productDescription=SALICYLIC+ACID+99%25+500G&vendorId=VN00024248&countryCode=US&language=en SDS] | ||
| − | + | ==Physical and Chemical Properties== | |
| − | The fluorescence of salicylic acid changes with pH. It is colorless below pH=2.5 and blue fluorescent above 3.5. | + | * Soluble in hot water, ethanol, acetone, ether. |
| + | * The fluorescence of salicylic acid changes with pH. It is colorless below pH=2.5 and blue fluorescent above 3.5. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 157-161 | + | | 157-161 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.443 | + | | 1.443 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 36: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 211 | + | | 211 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 872 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 872 | ||
Latest revision as of 12:19, 28 June 2022
Description
A white crystalline powder. Salicylic acid occurs naturally in wintergreen leaves and the bark of sweet birch. It is prepared synthetically for used in the manufacture of aspirin and dyestuffs. Salicylic acid is also used as a preservative and fungicide in pastes.
Synonyms and Related Terms
2-hydroxybenzoic acid; Keralyt; Occlusal; Verrugon; o-hydroxybenzoic acid
Risks
- Combustible.
- Toxic by ingestion of large amounts.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in hot water, ethanol, acetone, ether.
- The fluorescence of salicylic acid changes with pH. It is colorless below pH=2.5 and blue fluorescent above 3.5.
| Composition | C6H4(OH)(COOH) |
|---|---|
| CAS | 69-72-7 |
| Melting Point | 157-161 C |
| Density | 1.443 g/ml |
| Molecular Weight | mol. wt. = 138.1 |
| Boiling Point | 211 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 872
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8484
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: The fluorescence of salicylic acid changes with pH. It is colorless below pH=2.5 and blue fluorescent above 3.5.
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998