Difference between revisions of "Calcium nitrate"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
A white, deliquescent solid that is a strong oxidizing agent. Calcium nitrate was first produced commercially in Notodden, Norway in 1905. It is used in pyrotechnics, explosives, and match heads. Calcium nitrate has been identified as a deleterious accretion in wall paintings (Piqué et al 1992). The efflorescence also occurs when manure or other nitrogeneous compounds contact limestone in a dry environment. | A white, deliquescent solid that is a strong oxidizing agent. Calcium nitrate was first produced commercially in Notodden, Norway in 1905. It is used in pyrotechnics, explosives, and match heads. Calcium nitrate has been identified as a deleterious accretion in wall paintings (Piqué et al 1992). The efflorescence also occurs when manure or other nitrogeneous compounds contact limestone in a dry environment. | ||
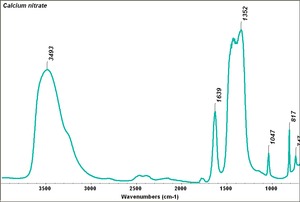
| − | + | [[[SliderGallery rightalign|Calcium nitrate.TIF~FTIR (MFA)]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
calcium dinitrate; lime nitrate; nitrocalcite; lime saltpeter; Norwegian saltpeter (Norgessalpeter); air saltpeter; calciumnitrat (Dan.); Kalksalpeter (Nor.); CalciNit | calcium dinitrate; lime nitrate; nitrocalcite; lime saltpeter; Norwegian saltpeter (Norgessalpeter); air saltpeter; calciumnitrat (Dan.); Kalksalpeter (Nor.); CalciNit | ||
| − | + | ==Risks== | |
| − | == | + | * Fire risk in contact with organic compounds. |
| + | * Integrachem: [http://www.integrachem.com/msds/C094_25358_101.pdf SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in water, methanol, ethanol, acetone. pH = 6.0 (5% solution) | Soluble in water, methanol, ethanol, acetone. pH = 6.0 (5% solution) | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 42-45 (hydrated) | + | | 42-45 C (hydrated) |
|- | |- | ||
| − | ! scope="row"| Density | + | ! scope="row"| Density (g/ml) |
| 1.82 (hydrated); 2.36 (dry) | | 1.82 (hydrated); 2.36 (dry) | ||
|- | |- | ||
| Line 31: | Line 34: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * F.Piqué, L.Dei, E.Ferroni "Physicochemical Aspects of the Deliquescence of Calcium Nitrate and Its Implications for Wall Painting Conservation" ''Studies in Conservation'' 37:217-227, 1992. | |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 49: | Line 44: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1729 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1729 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Calcium_nitrate (Accessed Jan. 15, 2006) |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 13:16, 18 May 2022
Description
A white, deliquescent solid that is a strong oxidizing agent. Calcium nitrate was first produced commercially in Notodden, Norway in 1905. It is used in pyrotechnics, explosives, and match heads. Calcium nitrate has been identified as a deleterious accretion in wall paintings (Piqué et al 1992). The efflorescence also occurs when manure or other nitrogeneous compounds contact limestone in a dry environment.
Synonyms and Related Terms
calcium dinitrate; lime nitrate; nitrocalcite; lime saltpeter; Norwegian saltpeter (Norgessalpeter); air saltpeter; calciumnitrat (Dan.); Kalksalpeter (Nor.); CalciNit
Risks
- Fire risk in contact with organic compounds.
- Integrachem: SDS
Physical and Chemical Properties
Soluble in water, methanol, ethanol, acetone. pH = 6.0 (5% solution)
| Composition | Ca(NO3)2 - 4H2O |
|---|---|
| CAS | 10124-37-5 (Anhydrous) 13477-34-4 (Tetrahydrate) |
| Melting Point | 42-45 C (hydrated) |
| Density (g/ml) | 1.82 (hydrated); 2.36 (dry) |
| Molecular Weight | mol. wt. = 236.15 |
Resources and Citations
- F.Piqué, L.Dei, E.Ferroni "Physicochemical Aspects of the Deliquescence of Calcium Nitrate and Its Implications for Wall Painting Conservation" Studies in Conservation 37:217-227, 1992.
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1729
- Wikipedia: http://en.wikipedia.org/wiki/Calcium_nitrate (Accessed Jan. 15, 2006)
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998