Difference between revisions of "Benzoyl peroxide"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white, crystalline solid. Benzoyl peroxide is an organic peroxide that is primarily used as a [ | + | A white, crystalline solid. Benzoyl peroxide is an organic peroxide that is primarily used as a [[catalyst]] for polymerization reactions, such as the curing of [[Bio-Plastic|Bio-Plastic®]] polyester resin. Benzoyl peroxide is also used to bleach [[flour]], [[fat|fats]], [[oil|oils]], and [[wax|waxes]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
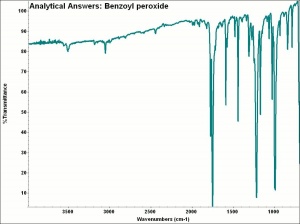
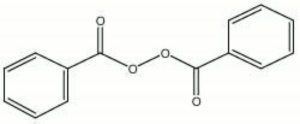
[[[SliderGallery rightalign|aaiBENZ_PER.jpg~FTIR|benzoyl peroxide.jpg~Chemical structure]]] | [[[SliderGallery rightalign|aaiBENZ_PER.jpg~FTIR|benzoyl peroxide.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | Flammable and explosive above 105C. Highly toxic by inhalation. Slightly toxic by skin contact and toxic by eye contact. | ||
| + | |||
| + | ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC211780050&productDescription=BENZOYL+PEROXIDE%2C+75%25%2C+R+5GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in most organic solvents. Slightly soluble in ethanol, oils and water. | Soluble in most organic solvents. Slightly soluble in ethanol, oils and water. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 103-105 | + | | 103-105 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.3340 | + | | 1.3340 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 36: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 49: | Line 48: | ||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | * Website | + | * Website: www.haz-map.com/alleric.htm |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:31, 4 May 2022
Description
A white, crystalline solid. Benzoyl peroxide is an organic peroxide that is primarily used as a Catalyst for polymerization reactions, such as the curing of Bio-Plastic® polyester resin. Benzoyl peroxide is also used to bleach Flour, fats, oils, and waxes.
Synonyms and Related Terms
benzoperoxide; dibenzoyl peroxide; benzoyl superoxide; Acetoxyl; Acnegel; Benoxyl; Oxy-5; Panoxyl; Persa-gel; Theraderm
Risks
Flammable and explosive above 105C. Highly toxic by inhalation. Slightly toxic by skin contact and toxic by eye contact.
ThermoFisher: SDS
Physical and Chemical Properties
Soluble in most organic solvents. Slightly soluble in ethanol, oils and water.
| Composition | (C6H5CO)2O2 |
|---|---|
| CAS | 94-36-0 |
| Melting Point | 103-105 C |
| Density | 1.3340 g/ml |
| Molecular Weight | mol. wt. = 242.2 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1159
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Website: www.haz-map.com/alleric.htm