Difference between revisions of "Cholesterol"
Jump to navigation
Jump to search
(username removed) |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 3: | Line 3: | ||
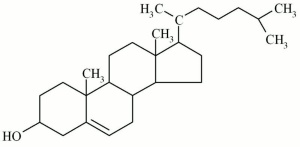
A white, waxy sterol that occurs in all animal tissues. Cholesterol provides protection for the skin and nerve cells. It is obtained commercially from lanolin and is used as an emollient in cosmetics, hair conditioners, and pharmaceuticals. | A white, waxy sterol that occurs in all animal tissues. Cholesterol provides protection for the skin and nerve cells. It is obtained commercially from lanolin and is used as an emollient in cosmetics, hair conditioners, and pharmaceuticals. | ||
| − | + | [[[SliderGallery rightalign|Cholesterolf5.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
cholest-5-en-3-ol; cholesterin; Cholesterin (Deut.); cholestérol (Fr.); colesterol (Esp., Port.); colesterolo (It.); cholesterol (Ned., Pol.); kolesterol (Sven.) | cholest-5-en-3-ol; cholesterin; Cholesterin (Deut.); cholestérol (Fr.); colesterol (Esp., Port.); colesterolo (It.); cholesterol (Ned., Pol.); kolesterol (Sven.) | ||
| − | [ | + | == Risks == |
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AAA1147018&productDescription=CHOLESTEROL%2C+99%2B%25+%28ASSAY%29+50G&vendorId=VN00024248&countryCode=US&language=en SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in ether, chloroform, benzene, pyridine, oils and fats. Insoluble in water. Gives an intense red color with rosaniline in chloroform solution. | Soluble in ether, chloroform, benzene, pyridine, oils and fats. Insoluble in water. Gives an intense red color with rosaniline in chloroform solution. | ||
| Line 23: | Line 24: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 148.5 | + | | 148.5 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.067 | + | | 1.067 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 32: | Line 33: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 360 (dec) | + | | 360 C (dec) |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Cholesterol (Accessed Oct. 18, 2005) |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:48, 29 May 2022
Description
A white, waxy sterol that occurs in all animal tissues. Cholesterol provides protection for the skin and nerve cells. It is obtained commercially from lanolin and is used as an emollient in cosmetics, hair conditioners, and pharmaceuticals.
Synonyms and Related Terms
cholest-5-en-3-ol; cholesterin; Cholesterin (Deut.); cholestérol (Fr.); colesterol (Esp., Port.); colesterolo (It.); cholesterol (Ned., Pol.); kolesterol (Sven.)
Risks
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in ether, chloroform, benzene, pyridine, oils and fats. Insoluble in water. Gives an intense red color with rosaniline in chloroform solution.
| Composition | C27H45OH |
|---|---|
| CAS | 57-88-5 |
| Melting Point | 148.5 C |
| Density | 1.067 g/ml |
| Molecular Weight | mol. wt. = 386.66 |
| Boiling Point | 360 C (dec) |
Resources and Citations
- Wikipedia: http://en.wikipedia.org/wiki/Cholesterol (Accessed Oct. 18, 2005)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry # 2256
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 881