Difference between revisions of "Dinitrobenzene, ortho"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Flat, prismatic crystals. Dinitrobenzene was discovered in 1841 by Sainte Claire Deville. It is prepared from [ | + | Flat, prismatic crystals. Dinitrobenzene was discovered in 1841 by Sainte Claire Deville. It is prepared from [[benzene]] treated with [[sulfuric acid]] and fuming [[nitric acid]]. |
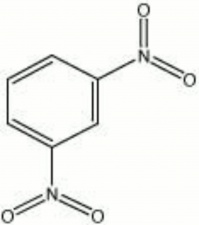
| − | + | [[[SliderGallery rightalign|dinitrobenzene, ortho.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
1,3-dinitrobenzene | 1,3-dinitrobenzene | ||
| − | + | == Risks == | |
| − | + | * Toxic by ingestion. | |
| + | * Contact cause burns and irritation. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/07316.htm MSDS] | ||
| + | ==Physical and Chemical Properties== | ||
Soluble in ether, benzene, chloroform, ethyl acetate, and hot alcohol | Soluble in ether, benzene, chloroform, ethyl acetate, and hot alcohol | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 118 | + | | 118 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.57 | + | | 1.57 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 34: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 319 | + | | 319 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #3273; bp=319 C, mp = 118C, density = 1.57 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #3273; bp=319 C, mp = 118C, density = 1.57 | ||
Latest revision as of 13:37, 21 July 2022
Description
Flat, prismatic crystals. Dinitrobenzene was discovered in 1841 by Sainte Claire Deville. It is prepared from Benzene treated with Sulfuric acid and fuming Nitric acid.
Synonyms and Related Terms
1,3-dinitrobenzene
Risks
- Toxic by ingestion.
- Contact cause burns and irritation.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ether, benzene, chloroform, ethyl acetate, and hot alcohol
| Composition | C6H4(NO2)2 |
|---|---|
| CAS | 99-65-0 |
| Melting Point | 118 C |
| Density | 1.57 g/ml |
| Molecular Weight | mol. wt. = 168.11 |
| Boiling Point | 319 C |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #3273; bp=319 C, mp = 118C, density = 1.57
- MSDS Sheet Comment: Fisher Scientific; bp = 88-90C, mp= 297 C, density 1.36
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876 Comment: p. 356