Difference between revisions of "Sodium citrate"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white crystalline or granular material. Sodium citrate is used as a [ | + | A white crystalline or granular material. Sodium citrate is used as a [[buffer|buffering agent]] in photographic solutions. It is also used as a [[chelating%20agent|chelating agent]] to remove soluble metals ions from solutions. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
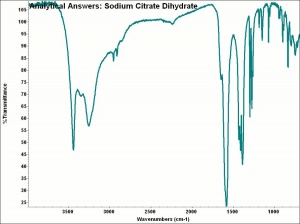
[[[SliderGallery rightalign|aaiNA_CITRA.jpg~FTIR|sodium citrate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|aaiNA_CITRA.jpg~FTIR|sodium citrate.jpg~Chemical structure]]] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in water forming a solution with a pH of about 8. Insoluble in ethanol. | Soluble in water forming a solution with a pH of about 8. Insoluble in ethanol. | ||
| Line 25: | Line 25: | ||
|} | |} | ||
| − | == | + | == Risks == |
| − | Combustible. Contact may cause irritation. | + | * Combustible. |
| + | * Contact may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=BP327500&productDescription=SODIUM+CITRATE.2H20+500G&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* Marie Svoboda, Conservation Survey Index, unpublished, 1997 | * Marie Svoboda, Conservation Survey Index, unpublished, 1997 | ||
Latest revision as of 15:27, 1 June 2022
Description
A white crystalline or granular material. Sodium citrate is used as a buffering agent in photographic solutions. It is also used as a Chelating agent to remove soluble metals ions from solutions.
Synonyms and Related Terms
trisodium citrate; citrosodine; Citnatrin; Urisal
Physical and Chemical Properties
Soluble in water forming a solution with a pH of about 8. Insoluble in ethanol.
| Composition | Na3C6H5O7 - 2H2O |
|---|---|
| CAS | 68-04-2 |
| Molecular Weight | mol. wt. = 258.1 |
Risks
- Combustible.
- Contact may cause irritation.
- ThermoFisher: SDS
Resources and Citations
- Marie Svoboda, Conservation Survey Index, unpublished, 1997
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 455
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 8746
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997