Difference between revisions of "Tungsten"
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | Hard, gray, metallic element. Tungsten was discovered in 1783 by Juan Jose and Fausto de Elhuyar of Spain. It has an abundance of 1.5 ppm in the earth's crust and is primarily found in scheelite (CaWO4) and wolframite [(Fe,Mn)WO4] ores. Tungsten is mined in China, Malaya, Mexico, Canada, Bolivia, Peru, and the U.S (Texas, Alaska). Metallic tungsten has the highest melting point of all metals. It is added to steel alloys to increase strength and corrosion resistance. Metallic tungsten is used as wires and filaments in [ | + | Hard, gray, metallic element. Tungsten was discovered in 1783 by Juan Jose and Fausto de Elhuyar of Spain. It has an abundance of 1.5 ppm in the earth's crust and is primarily found in scheelite (CaWO4) and wolframite [(Fe,Mn)WO4] ores. Tungsten is mined in China, Malaya, Mexico, Canada, Bolivia, Peru, and the U.S (Texas, Alaska). Metallic tungsten has the highest melting point of all metals. It is added to steel alloys to increase strength and corrosion resistance. Metallic tungsten is used as wires and filaments in [[electric%20lamp|Electric lamps]], and furnaces. It is also used for thermocouples, spark plugs, electrical contacts, and cutting tools. |
[[File:TungstenMetalemr1.jpg|thumb|Tungsten metal]] | [[File:TungstenMetalemr1.jpg|thumb|Tungsten metal]] | ||
| Line 11: | Line 11: | ||
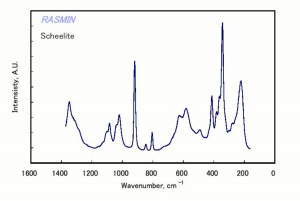
[[[SliderGallery rightalign|scheeliteRS.jpg~Raman]]] | [[[SliderGallery rightalign|scheeliteRS.jpg~Raman]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Powdered tungsten is flammable and may ignite spontaneously (pyrophoric). | ||
| + | * Contact may cause irritation | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/99069.htm MSDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Unaffected by mineral acids except for hydrofluoric mixed with nitric. | Unaffected by mineral acids except for hydrofluoric mixed with nitric. | ||
| Line 27: | Line 33: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 3410 | + | | 3410 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 19.3 | + | | 19.3 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 36: | Line 42: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 5927 | + | | 5927 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "tungsten" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "tungsten" [Accessed 20 Sept. 2005]. |
* ''Chemical & Engineering News'', American Chemical Society, Washington DC, 81 (36) , Sept. 8, 2003 Comment: Rick Lowden, p. 142: isolated in 1783 by Spanish chemists Juan Jose and Fausto Elhuyar | * ''Chemical & Engineering News'', American Chemical Society, Washington DC, 81 (36) , Sept. 8, 2003 Comment: Rick Lowden, p. 142: isolated in 1783 by Spanish chemists Juan Jose and Fausto Elhuyar | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Tungsten (Accessed Sept. 20, 2005) |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 71: | Line 67: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * | + | * Web Elements at http://www.webelements.com/webelements/elements/text/W/key.html states discovered by Fausto and Juan Jose de Elhuyar in 1783 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:21, 17 March 2025
Description
Hard, gray, metallic element. Tungsten was discovered in 1783 by Juan Jose and Fausto de Elhuyar of Spain. It has an abundance of 1.5 ppm in the earth's crust and is primarily found in scheelite (CaWO4) and wolframite [(Fe,Mn)WO4] ores. Tungsten is mined in China, Malaya, Mexico, Canada, Bolivia, Peru, and the U.S (Texas, Alaska). Metallic tungsten has the highest melting point of all metals. It is added to steel alloys to increase strength and corrosion resistance. Metallic tungsten is used as wires and filaments in Electric lamps, and furnaces. It is also used for thermocouples, spark plugs, electrical contacts, and cutting tools.
Synonyms and Related Terms
W; Wolfraam (Ned.); tungstène (Fr.); Wolfram (Deut., Dan.); tungsteno (It.); wolframio (It., Esp.); tungsténio (Port.); volfram (Sven.) ; scheelite (CaWO4); wolframite [(Fe,Mn)WO4]
Risks
- Powdered tungsten is flammable and may ignite spontaneously (pyrophoric).
- Contact may cause irritation
- Fisher Scientific: MSDS
Physical and Chemical Properties
Unaffected by mineral acids except for hydrofluoric mixed with nitric.
| Composition | W (atomic no. 74) |
|---|---|
| CAS | 7440-33-7 |
| Mohs Hardness | 6.5-7.5 |
| Melting Point | 3410 C |
| Density | 19.3 g/ml |
| Molecular Weight | atomic wt = 183.85 |
| Boiling Point | 5927 C |
Resources and Citations
- Encyclopedia Britannica, http://www.britannica.com Comment: "tungsten" [Accessed 20 Sept. 2005].
- Chemical & Engineering News, American Chemical Society, Washington DC, 81 (36) , Sept. 8, 2003 Comment: Rick Lowden, p. 142: isolated in 1783 by Spanish chemists Juan Jose and Fausto Elhuyar
- Wikipedia: http://en.wikipedia.org/wiki/Tungsten (Accessed Sept. 20, 2005)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9945; states discovered by Scheele in 1781 and isolated by J and F de Elhuyar
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 827
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Web Elements at http://www.webelements.com/webelements/elements/text/W/key.html states discovered by Fausto and Juan Jose de Elhuyar in 1783