Difference between revisions of "Anglesite"
(username removed) |
|||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A naturally occurring mineral composed of [ | + | A naturally occurring mineral composed of [[lead%20sulfate|lead sulfate]]. Anglesite occurs naturally as an oxidation product of [[galena|galena]] (lead sulfide). First recognized as a mineral in 1783 by Dr. Withering in the Anglesey copper mine, anglesite is now mined in England, Wales (Anglesey), Scotland (Leadhills), Italy (Sardinia), Australia, Mexico (Chihuahua), and the United States (Pennsylvania, Idaho, Nevada). Anglesite is a lustrous white to colorless stone that can be transparent or opaque. It is primarily used as an ore source for lead. White crystals of anglesite also occur as a lead corrosion product (Selwyn 1996). |
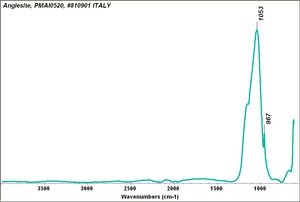
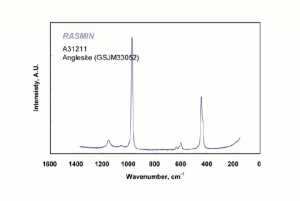
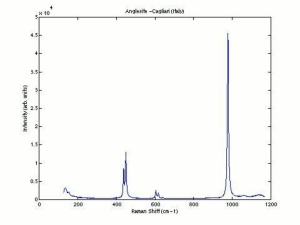
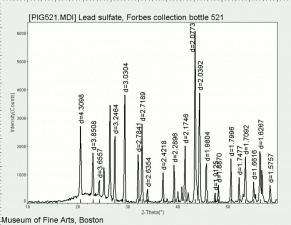
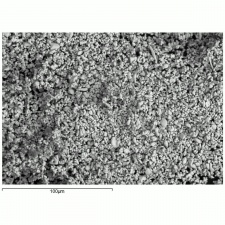
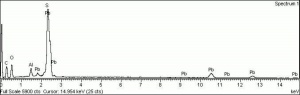
| − | + | [[[SliderGallery rightalign|Anglesite PMA.TIF~FTIR (PMA)|anglesiteRS.jpg~Raman (RASMIN)|Anglesiteitaly1.jpg~Raman (U of Parma)|PIG521.jpg~XRD (MFA)|f521sem.jpg~SEM (MFA)|f521edsbw.jpg~EDS (MFA)]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
lead sulfate; lead vitriol; anglésite (Fr.); anglesita (Esp.); anglesite (Port.); Anglesit (Deut.); anglesiet (Ned.) | lead sulfate; lead vitriol; anglésite (Fr.); anglesita (Esp.); anglesite (Port.); Anglesit (Deut.); anglesiet (Ned.) | ||
| − | + | == Ricks == | |
| − | == | + | Toxic by inhalation, ingestion and skin contact. |
| + | == Physical and Chemical Properties == | ||
Slightly soluble in water. | Slightly soluble in water. | ||
| − | Orthorhombic systems usually occurs as thin tabular crystals. Cleavage is good in one direction, distinct in a second direction. | + | * Orthorhombic systems usually occurs as thin tabular crystals. |
| − | + | * Cleavage is good in one direction, distinct in a second direction. | |
| − | Fracture - conchoidal. Luster = adamantine. Streak = white to grayish. | + | * Fracture - conchoidal. |
| + | * Luster = adamantine. | ||
| + | * Streak = white to grayish. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 27: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 1170 | + | | 1170 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 6.12-6.39 | + | | 6.12-6.39 g/ml |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 45: | Line 40: | ||
[[media:download_file_533.pdf|Characteristics of Common White Pigments]] | [[media:download_file_533.pdf|Characteristics of Common White Pigments]] | ||
| + | ==Resources and Citations== | ||
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Anglesite.shtml Anglesite] | |
| − | |||
* Henry Hodges, ''Artifacts: An Introduction to Early Materials and Technology'', Ronald P. Frye, Kingston, Canada, 1988 | * Henry Hodges, ''Artifacts: An Introduction to Early Materials and Technology'', Ronald P. Frye, Kingston, Canada, 1988 | ||
| Line 65: | Line 60: | ||
* L. Selwyn, 'Historical Silver: Storage, Display and Tarnish Removal', ''J.IIC-GC'', 15, 1990 | * L. Selwyn, 'Historical Silver: Storage, Display and Tarnish Removal', ''J.IIC-GC'', 15, 1990 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Anglesite |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 09:20, 8 December 2022
Description
A naturally occurring mineral composed of Lead sulfate. Anglesite occurs naturally as an oxidation product of Galena (lead sulfide). First recognized as a mineral in 1783 by Dr. Withering in the Anglesey copper mine, anglesite is now mined in England, Wales (Anglesey), Scotland (Leadhills), Italy (Sardinia), Australia, Mexico (Chihuahua), and the United States (Pennsylvania, Idaho, Nevada). Anglesite is a lustrous white to colorless stone that can be transparent or opaque. It is primarily used as an ore source for lead. White crystals of anglesite also occur as a lead corrosion product (Selwyn 1996).
Synonyms and Related Terms
lead sulfate; lead vitriol; anglésite (Fr.); anglesita (Esp.); anglesite (Port.); Anglesit (Deut.); anglesiet (Ned.)
Ricks
Toxic by inhalation, ingestion and skin contact.
Physical and Chemical Properties
Slightly soluble in water.
- Orthorhombic systems usually occurs as thin tabular crystals.
- Cleavage is good in one direction, distinct in a second direction.
- Fracture - conchoidal.
- Luster = adamantine.
- Streak = white to grayish.
| Composition | PbSO4 |
|---|---|
| Mohs Hardness | 2.5 - 3.0 |
| Melting Point | 1170 C |
| Density | 6.12-6.39 g/ml |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Mineralogy Database: Anglesite
- Henry Hodges, Artifacts: An Introduction to Early Materials and Technology, Ronald P. Frye, Kingston, Canada, 1988
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 414
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: anglesite [Accessed December 4, 2001].
- L. Selwyn, 'Historical Silver: Storage, Display and Tarnish Removal', J.IIC-GC, 15, 1990
- Wikipedia: http://en.wikipedia.org/wiki/Anglesite