Difference between revisions of "Chrome green"
(username removed) |
|||
| (10 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:350 chrome green.jpg|thumb|Chrome green]] | [[File:350 chrome green.jpg|thumb|Chrome green]] | ||
== Description == | == Description == | ||
| + | [[File:chromegreen C100x.jpg|thumb|Chrome green at 100x (Visible light on left / UV light on right)]] | ||
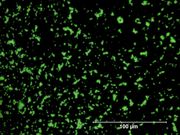
| + | [[File:32_Chrome_green_500X.jpg|thumb|Chrome green at 500x]] | ||
| + | A term used for many different green compounds containing [[chromium]]. As early as 1809, chromium oxide, or chromia, was used as an enamel in porcelain factories. It was later sold as a stable, transparent green pigment in 1838 by Pannetier and Binet in Paris. However, the term also became used for [[viridian]] and later for a pigment mixture prepared with chrome yellow ([[lead chromate]]) and [[Prussian blue]]. Originally chrome green was used in artists paints, inks and glasses, but its used decreased to just house paints and industrial products. | ||
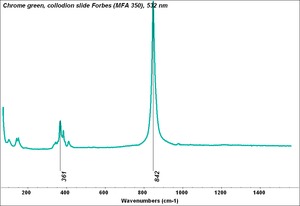
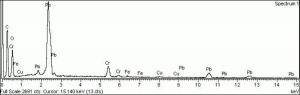
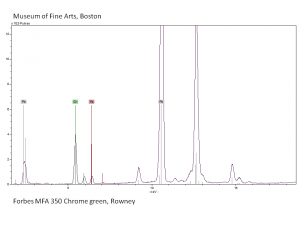
| − | + | [[[SliderGallery rightalign|Chrome green, collodion slide Forbes (MFA 350), 532 nm.TIF~Raman (MFA)|f350sem.jpg~SEM|f350edsbw.jpg~EDS|Slide10 FC350.PNG~XRF (MFA)]]] | |
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | Pigment Green 15; CI 77600; verde cromo (Esp.); vert de chrome (Fr.); Zinnobergrün (Deut.); Chromoxydgrün (Deut.); prasino toy chromioy (Gr.); verde cromo (It.); chromaatgroen (Ned.); verde de crómio (Port.); cinnabar green; green vermilion; Victoria green; Prussian green; bronze green; Milori green; Brunswick green; nitrate green; royal green; zinnober green; oil green; | + | Pigment Green 15; CI 77600; verde cromo (Esp.); vert de chrome (Fr.); Zinnobergrün (Deut.); Chromoxydgrün (Deut.); prasino toy chromioy (Gr.); verde cromo (It.); chromaatgroen (Ned.); verde de crómio (Port.); oxide of chromium; cinnabar green; green vermilion; Victoria green; Prussian green; bronze green; Milori green; Brunswick green; nitrate green; royal green; zinnober green; oil green; |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | == Risks == | |
| − | |||
| − | |||
| − | |||
| + | * Toxic by inhalation, ingestion and skin absorption. | ||
| + | * Human carcinogen and teratogen; suspected mutagen. | ||
| + | * Solomon Colors: [https://www.solomoncolors.com/documents/solomon/sds/Green-Chrome-Oxide-SDS.pdf SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
| + | For mixture of chrome yellow and Prussian blue: | ||
| + | * Turns blue with exposure to strong light or acids. | ||
| + | * Turns dark orange with exposure to alkalis. | ||
| + | * Individual blue and yellow particles are small and cannot usually be distinguish microscopically. | ||
| + | ==Resources and Citations== | ||
| + | * Pigments Through the Ages: [https://www.webexhibits.org/pigments/indiv/history/viridian.html Viridian] Accessed March 2025 | ||
| + | * Natural Pigments: [https://www.naturalpigments.com/chromium-oxide-green-dispersion.html#:~:text=Origin%20and%20History&text=As%20early%20as%201809%2C%20chromium,been%20identified%20on%20a%20J.M.W. Chromium oxide] | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density=4.06, ref index=~2.4 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density=4.06, ref index=~2.4 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 611 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 611 | ||
| − | |||
* Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Chromium(III)_oxide Chromium (III) oxide] Accessed March 2025 | |
* Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | ||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | ||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:29, 15 March 2025
Description
A term used for many different green compounds containing Chromium. As early as 1809, chromium oxide, or chromia, was used as an enamel in porcelain factories. It was later sold as a stable, transparent green pigment in 1838 by Pannetier and Binet in Paris. However, the term also became used for Viridian and later for a pigment mixture prepared with chrome yellow (Lead chromate) and Prussian blue. Originally chrome green was used in artists paints, inks and glasses, but its used decreased to just house paints and industrial products.
Synonyms and Related Terms
Pigment Green 15; CI 77600; verde cromo (Esp.); vert de chrome (Fr.); Zinnobergrün (Deut.); Chromoxydgrün (Deut.); prasino toy chromioy (Gr.); verde cromo (It.); chromaatgroen (Ned.); verde de crómio (Port.); oxide of chromium; cinnabar green; green vermilion; Victoria green; Prussian green; bronze green; Milori green; Brunswick green; nitrate green; royal green; zinnober green; oil green;
Risks
- Toxic by inhalation, ingestion and skin absorption.
- Human carcinogen and teratogen; suspected mutagen.
- Solomon Colors: SDS
Physical and Chemical Properties
For mixture of chrome yellow and Prussian blue:
- Turns blue with exposure to strong light or acids.
- Turns dark orange with exposure to alkalis.
- Individual blue and yellow particles are small and cannot usually be distinguish microscopically.
Resources and Citations
- Pigments Through the Ages: Viridian Accessed March 2025
- Natural Pigments: Chromium oxide
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density=4.06, ref index=~2.4
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 611
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Wikipedia: Chromium (III) oxide Accessed March 2025
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"