Difference between revisions of "Red lead"
(username removed) |
|||
| (9 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:1994.187-SC58835.jpg|thumb|Storage basket with red lead decorations<br>MFA# 1994.187]] | ||
[[File:199 red lead.jpg|thumb|Red lead]] | [[File:199 red lead.jpg|thumb|Red lead]] | ||
== Description == | == Description == | ||
| + | [[File:25_Red_lead_500X.jpg|thumb|Red lead at 500x transmitted light]] | ||
| + | [[File:25_Red_lead_500X_pol.jpg|thumb|Red lead at 500x polarized light]] | ||
| + | The common name for a heavy, opaque, orange-red pigment composed of [[lead tetroxide]]. Although chemically equivalent to the mineral minium, red lead has been synthetically prepared by roasting lead white (480 C) since before the 5th century BCE. Red lead has been found as a pigment on early objects from Egypt, China, Japan, India, Persia, and Rome. Red lead is no longer used as an artists color because is has poor light stability and poor working properties. Instead, red lead is used to color glass and ceramic glazes. Red lead paint was used as an anticorrosion primer for structural iron and steel until the late 20th century. | ||
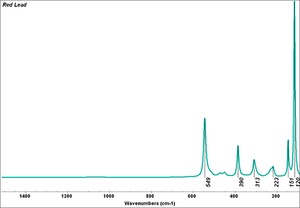
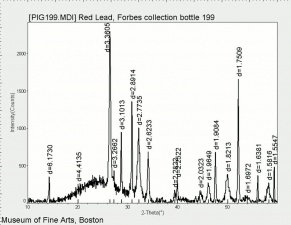
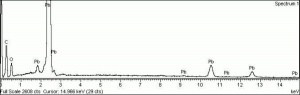
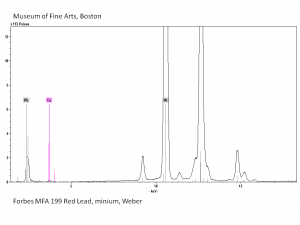
| − | + | [[[SliderGallery rightalign|Red Lead resize.tif~Raman (MFA)|PIG199.jpg~XRD|f199sem.jpg~SEM|f199edsbw.jpg~EDS|red lead.jpg~Chemical structure|Slide16 FC199.PNG~XRF]]] | |
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | red lead oxide; lead tetroxide; Pigment Red 105; CI 77578; minium (Fr., Lt.); minimium secondarium; plumbus plumbate; Mennige (Deut.); Bleimennige (Deut.); minium de plomb (Fr.); mønje (Dan.); minio (Esp., Gr.); minio (cerussa usta) (It.); minio di piombo (It.); entan (Jap.); tan (Jap.); loodmenie (Ned.); vermelho de chumbo (Port.); mínio (Port.); orange lead; Paris red; Saturn red; saturnine red; orange mineral; burnt white lead; red oxide of lead; mineral red; false sandarach; sandyx; syricum | + | Lead(II,IV) oxide; red lead oxide; lead tetroxide; Pigment Red 105; CI 77578; minium (Fr., Lt.); minimium secondarium; plumbus plumbate; Mennige (Deut.); Bleimennige (Deut.); minium de plomb (Fr.); mønje (Dan.); minio (Esp., Gr.); minio (cerussa usta) (It.); minio di piombo (It.); entan (Jap.); tan (Jap.); loodmenie (Ned.); vermelho de chumbo (Port.); mínio (Port.); orange lead; Paris red; Saturn red; saturnine red; orange mineral; burnt white lead; red oxide of lead; mineral red; false sandarach; sandyx; syricum |
| − | + | == Risks == | |
| − | == | + | * Toxic by inhalation or ingestion. |
| + | * Skin contact may cause irritation or ulcers. | ||
| + | * Carcinogen, teratogen, suspected mutagen. | ||
| + | * Discolored by exposure to light, hydrogen sulfide and acids. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC221110050&productDescription=LEAD%28II%2CIV%29+OXIDE+RED+98%25+5G&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
| − | Discolors with acids (nitric acid - brown, sulfuric acid and sulfides - black, hydrochloic acid - white). | + | * Discolors with acids (nitric acid - brown, sulfuric acid and sulfides - black, hydrochloic acid - white). |
| − | + | * Fine particles that are a transparent orange red. | |
| − | Fine particles that are a transparent orange red. Low birefringence. | + | * Low birefringence. |
{| class="wikitable" | {| class="wikitable" | ||
| Line 26: | Line 34: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 8.73 | + | | 8.73 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 35: | Line 43: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * ''Artists | + | * E. West-Fitzhugh, "Red Lead and Minium", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press; Cambridge, 1986 |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 71: | Line 57: | ||
* Robert Fournier, ''Illustrated Dictionary of Practical Pottery'', Chilton Book Company, Radnor, PA, 1992 | * Robert Fournier, ''Illustrated Dictionary of Practical Pottery'', Chilton Book Company, Radnor, PA, 1992 | ||
| − | * | + | * Pigments Through The Ages - http://webexhibits.org/pigments/indiv/technical/redlead.html - Crystal system: tetragonal Refractive index: 2.42 |
* R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | ||
| Line 81: | Line 67: | ||
* R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', ''Japanese Woodblock Prints'', Allen Memorial Art Museum, Oberlin College, Oberlin, 1984 | * R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', ''Japanese Woodblock Prints'', Allen Memorial Art Museum, Oberlin College, Oberlin, 1984 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Red_lead (Accessed Sept. 14, 2005) |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:52, 19 September 2022
Description
The common name for a heavy, opaque, orange-red pigment composed of Lead tetroxide. Although chemically equivalent to the mineral minium, red lead has been synthetically prepared by roasting lead white (480 C) since before the 5th century BCE. Red lead has been found as a pigment on early objects from Egypt, China, Japan, India, Persia, and Rome. Red lead is no longer used as an artists color because is has poor light stability and poor working properties. Instead, red lead is used to color glass and ceramic glazes. Red lead paint was used as an anticorrosion primer for structural iron and steel until the late 20th century.
Synonyms and Related Terms
Lead(II,IV) oxide; red lead oxide; lead tetroxide; Pigment Red 105; CI 77578; minium (Fr., Lt.); minimium secondarium; plumbus plumbate; Mennige (Deut.); Bleimennige (Deut.); minium de plomb (Fr.); mønje (Dan.); minio (Esp., Gr.); minio (cerussa usta) (It.); minio di piombo (It.); entan (Jap.); tan (Jap.); loodmenie (Ned.); vermelho de chumbo (Port.); mínio (Port.); orange lead; Paris red; Saturn red; saturnine red; orange mineral; burnt white lead; red oxide of lead; mineral red; false sandarach; sandyx; syricum
Risks
- Toxic by inhalation or ingestion.
- Skin contact may cause irritation or ulcers.
- Carcinogen, teratogen, suspected mutagen.
- Discolored by exposure to light, hydrogen sulfide and acids.
- ThermoFisher: SDS
Physical and Chemical Properties
- Discolors with acids (nitric acid - brown, sulfuric acid and sulfides - black, hydrochloic acid - white).
- Fine particles that are a transparent orange red.
- Low birefringence.
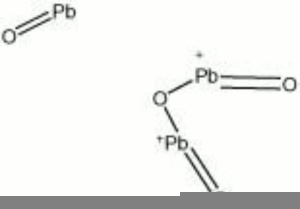
| Composition | Pb3O4 |
|---|---|
| CAS | 1314-41-6 |
| Density | 8.73 g/ml |
| Molecular Weight | mol. wt. = 685.60 |
| Refractive Index | 2.42 |
Resources and Citations
- E. West-Fitzhugh, "Red Lead and Minium", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press; Cambridge, 1986
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 657
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Robert Fournier, Illustrated Dictionary of Practical Pottery, Chilton Book Company, Radnor, PA, 1992
- Pigments Through The Ages - http://webexhibits.org/pigments/indiv/technical/redlead.html - Crystal system: tetragonal Refractive index: 2.42
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', Japanese Woodblock Prints, Allen Memorial Art Museum, Oberlin College, Oberlin, 1984
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Wikipedia: http://en.wikipedia.org/wiki/Red_lead (Accessed Sept. 14, 2005)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993