Difference between revisions of "Japan wax"
(username removed) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A pale yellow, soft vegetable wax obtained from the berries of the Japanese sumac tree (''Rhus verniciflua)'' and the Japanese wax tree, (''Rhus succedanea''). Japan wax is a byproduct of lacquer manufacture. It is not a true wax but a fat that contains 10-15% [ | + | A pale yellow, soft vegetable wax obtained from the berries of the Japanese sumac tree (''Rhus verniciflua)'' and the Japanese wax tree, (''Rhus succedanea''). Japan wax is a byproduct of lacquer manufacture. It is not a true wax but a fat that contains 10-15% [[palmitic acid]], [[stearic acid]], and [[oleic acid]] with about 1% japanic acid. Japan wax is sold in flat squares or disks and has a rancid odor. It is used in the manufacture of candles, pharmaceuticals and cosmetics. Japan wax is also used in floor waxes, furniture polishes, pastels, crayons, buffing compounds, metal lubricants, adhesives, and as a substitute for [[beeswax]]. |
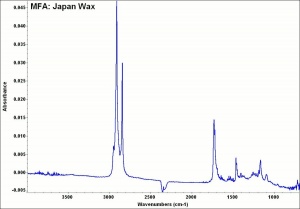
| − | + | [[[SliderGallery rightalign|Japan Wax 1933 Harvard plates 86.jpg~FTIR]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
''Rhus verniciflua'' (Japanese sumac tree); ''Rhus succedanea'' (Japanese wax tree); cera del Giappone (It); Japan tallow; sumac wax; sumach wax; vegetable wax | ''Rhus verniciflua'' (Japanese sumac tree); ''Rhus succedanea'' (Japanese wax tree); cera del Giappone (It); Japan tallow; sumac wax; sumach wax; vegetable wax | ||
| − | + | == Risks == | |
| − | == | + | Combustible. |
| − | + | == Physical and Chemical Properties == | |
| − | |||
| − | Iodine | + | * Soluble in benzene, ether, naphtha and alkalis. Insoluble in water or cold ethanol. |
| + | * Iodine value=4.5-12.6 | ||
| + | * Acid value=6-209 | ||
| + | * Saponification value=206.5-237.5 | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 48-56 | + | | 48-56 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.97-0.99 | + | | 0.97-0.99 g/ml |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_25.pdf|Properties of Natural Waxes]] |
| − | |||
| − | |||
| − | == | + | == Resources and Citations == |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 424; melting point = 51C, density = 0.975, saponification value = 220; iodine value = 12 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 424; melting point = 51C, density = 0.975, saponification value = 220; iodine value = 12 | ||
Latest revision as of 14:49, 31 August 2022
Description
A pale yellow, soft vegetable wax obtained from the berries of the Japanese sumac tree (Rhus verniciflua) and the Japanese wax tree, (Rhus succedanea). Japan wax is a byproduct of lacquer manufacture. It is not a true wax but a fat that contains 10-15% Palmitic acid, Stearic acid, and Oleic acid with about 1% japanic acid. Japan wax is sold in flat squares or disks and has a rancid odor. It is used in the manufacture of candles, pharmaceuticals and cosmetics. Japan wax is also used in floor waxes, furniture polishes, pastels, crayons, buffing compounds, metal lubricants, adhesives, and as a substitute for Beeswax.
Synonyms and Related Terms
Rhus verniciflua (Japanese sumac tree); Rhus succedanea (Japanese wax tree); cera del Giappone (It); Japan tallow; sumac wax; sumach wax; vegetable wax
Risks
Combustible.
Physical and Chemical Properties
- Soluble in benzene, ether, naphtha and alkalis. Insoluble in water or cold ethanol.
- Iodine value=4.5-12.6
- Acid value=6-209
- Saponification value=206.5-237.5
| Melting Point | 48-56 C |
|---|---|
| Density | 0.97-0.99 g/ml |
Comparisons
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 424; melting point = 51C, density = 0.975, saponification value = 220; iodine value = 12
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: melting point = 48-55C
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: melting point 50-52C
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982 Comment: melting point = 50-56C
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- A History of Technology, Charles Singer, E.J. Holmyard, A.R. Hall (eds.), Clarendon Press, Oxford, Volume 1: From Early times to Fall of Ancient Empires, 1954
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point=48-53C, density=0.975-0.993, iodine value=4.5-12.5, acid value=6-20, saponification value=206.5-237-5
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000